
 Copy to clipboard
Copy to clipboard 
Embody was granted FDA 510(k) clearance to market the TAPESTRY® Biointegrative Implant for tendon and ligament repair.
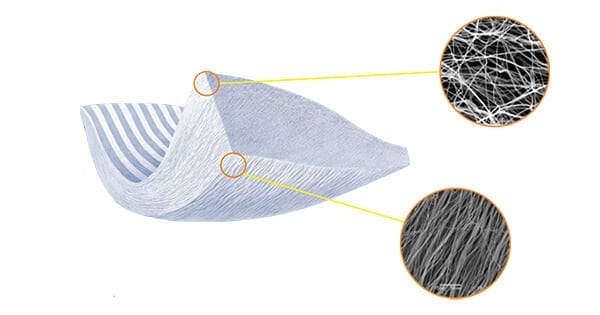
The outer surface of TAPESTRY comprises unaligned fibers designed for isotropic suture retention, strength and structural integrity. The inner surface features an aligned, porous surface with consistent microarchitecture to be surgically fixed to the native tendon.
With initial funding, Embody leveraged additive manufacturing and biofabrication to assemble collagen from the molecular scale to produce bioengineered medical devices specifically designed for soft tissue repair. A $9.3 million Series A funding round, closed in 2Q20, is supporting new hires and the planned 2H20 launch of TAPESTRY.
“This FDA clearance represents a major milestone in the advancement of Embody’s mission to improve outcomes for patients who suffer from tendon and ligament injuries,” said Jeff Conroy, CEO of Embody. “We believe TAPESTRY represents a significant advancement in collagen science and provides surgeons with a compelling solution with broad clinical utility for tendon repair procedures.”
Embody was granted FDA 510(k) clearance to market the TAPESTRY® Biointegrative Implant for tendon and ligament repair.
The outer surface of TAPESTRY comprises unaligned fibers designed for isotropic suture retention, strength and structural integrity. The inner surface features an aligned, porous surface with consistent microarchitecture to...
Embody was granted FDA 510(k) clearance to market the TAPESTRY® Biointegrative Implant for tendon and ligament repair.
The outer surface of TAPESTRY comprises unaligned fibers designed for isotropic suture retention, strength and structural integrity. The inner surface features an aligned, porous surface with consistent microarchitecture to be surgically fixed to the native tendon.
With initial funding, Embody leveraged additive manufacturing and biofabrication to assemble collagen from the molecular scale to produce bioengineered medical devices specifically designed for soft tissue repair. A $9.3 million Series A funding round, closed in 2Q20, is supporting new hires and the planned 2H20 launch of TAPESTRY.
“This FDA clearance represents a major milestone in the advancement of Embody’s mission to improve outcomes for patients who suffer from tendon and ligament injuries,” said Jeff Conroy, CEO of Embody. “We believe TAPESTRY represents a significant advancement in collagen science and provides surgeons with a compelling solution with broad clinical utility for tendon repair procedures.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







