
 Copy to clipboard
Copy to clipboard 
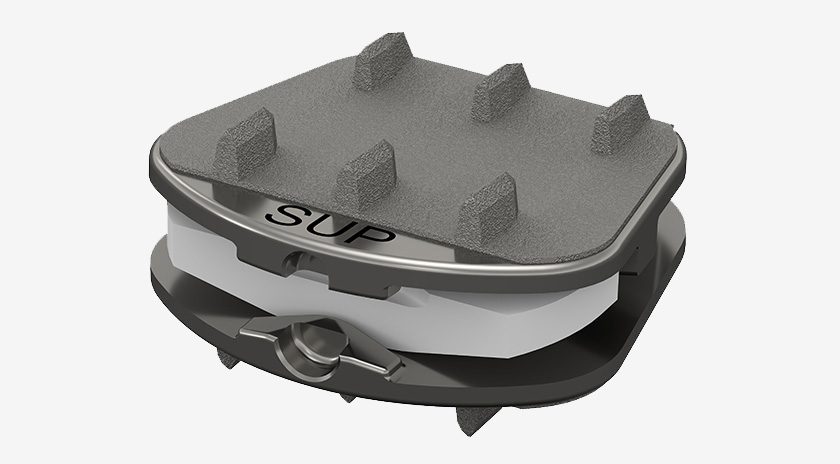
Synergy Spine Solutions has enrolled the first patient in its U.S. IDE 2-Level clinical trial.
The trial will evaluate the safety and effectiveness of the Synergy Disc artificial cervical disc compared to anterior cervical discectomy and fusion for the treatment of degenerative disc disease in subjects that are symptomatic at two contiguous vertebral levels from C3 to C7.
The Synergy Disc trial is a multi-center, prospective, non-randomized, historically controlled study that will be conducted on 200 patients at up to 25 sites. All patients in the study will receive the Synergy Disc. Patients will be evaluated preoperatively, at the time of surgery, immediately following surgery, at six weeks and three, six, 12 and 24 months after surgery. Follow-up will continue annually until the last patient reaches 24-month follow-up.
Josh Butters, CEO of Synergy Spine Solutions, said, “Beginning our 2-Level trial on the heels of completing enrollment in the 1-Level trial is another significant advance in our U.S. market access pathway, and puts us further down the path towards realizing our mission of expanding the surgeons and patients that have access to the Synergy Disc technology.”
Source: Synergy Spine Solutions
Synergy Spine Solutions has enrolled the first patient in its U.S. IDE 2-Level clinical trial.
The trial will evaluate the safety and effectiveness of the Synergy Disc artificial cervical disc compared to anterior cervical discectomy and fusion for the treatment of degenerative disc disease in subjects that are symptomatic at two contiguous...
Synergy Spine Solutions has enrolled the first patient in its U.S. IDE 2-Level clinical trial.
The trial will evaluate the safety and effectiveness of the Synergy Disc artificial cervical disc compared to anterior cervical discectomy and fusion for the treatment of degenerative disc disease in subjects that are symptomatic at two contiguous vertebral levels from C3 to C7.
The Synergy Disc trial is a multi-center, prospective, non-randomized, historically controlled study that will be conducted on 200 patients at up to 25 sites. All patients in the study will receive the Synergy Disc. Patients will be evaluated preoperatively, at the time of surgery, immediately following surgery, at six weeks and three, six, 12 and 24 months after surgery. Follow-up will continue annually until the last patient reaches 24-month follow-up.
Josh Butters, CEO of Synergy Spine Solutions, said, “Beginning our 2-Level trial on the heels of completing enrollment in the 1-Level trial is another significant advance in our U.S. market access pathway, and puts us further down the path towards realizing our mission of expanding the surgeons and patients that have access to the Synergy Disc technology.”
Source: Synergy Spine Solutions

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







