
 Copy to clipboard
Copy to clipboard 
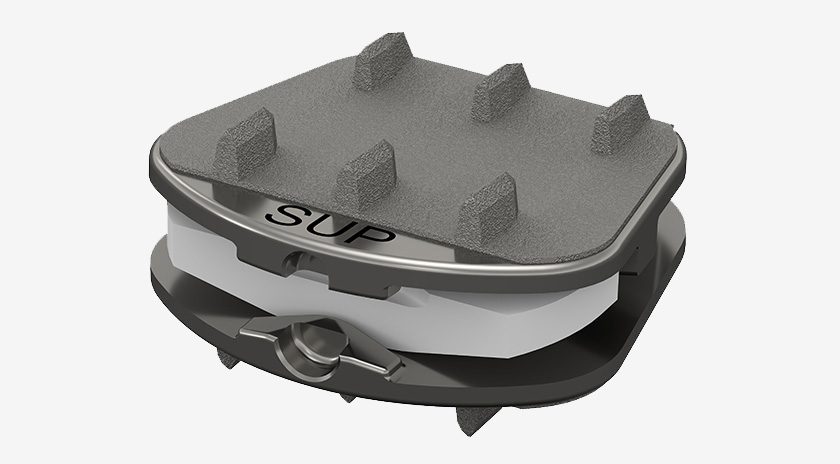
Synergy Spine Solutions completed enrollment in its first-ever Investigational Device Exemption (IDE) clinical trial.
The 1-Level Synergy Disc IDE clinical trial enrolled 175 patients in its multi-center, prospective, historically-controlled pivotal trial comparing the safety and effectiveness of the Synergy Disc to anterior cervical discectomy and fusion in patients with one-level symptomatic cervical degenerative disc disease. The study is being conducted at 20 sites across the United States and will be used to support a premarket approval application in the U.S.
Josh Butters, Synergy Spine Solutions CEO, said, “We are excited to continue following these patients, further building Synergy’s clinical evidence, and advancing our pre-market approval strategy for the Synergy Disc in the U.S.”
Source: Synergy Spine Solutions
Synergy Spine Solutions completed enrollment in its first-ever Investigational Device Exemption (IDE) clinical trial.
The 1-Level Synergy Disc IDE clinical trial enrolled 175 patients in its multi-center, prospective, historically-controlled pivotal trial comparing the safety and effectiveness of the Synergy Disc to anterior cervical...
Synergy Spine Solutions completed enrollment in its first-ever Investigational Device Exemption (IDE) clinical trial.
The 1-Level Synergy Disc IDE clinical trial enrolled 175 patients in its multi-center, prospective, historically-controlled pivotal trial comparing the safety and effectiveness of the Synergy Disc to anterior cervical discectomy and fusion in patients with one-level symptomatic cervical degenerative disc disease. The study is being conducted at 20 sites across the United States and will be used to support a premarket approval application in the U.S.
Josh Butters, Synergy Spine Solutions CEO, said, “We are excited to continue following these patients, further building Synergy’s clinical evidence, and advancing our pre-market approval strategy for the Synergy Disc in the U.S.”
Source: Synergy Spine Solutions

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







