





Curiteva announced a limited commercial release utilizing its recently FDA-cleared Inspire Porous PEEK HAFUSE Cervical Interbody. Full commercial U.S. launch is slated for later in 2023.
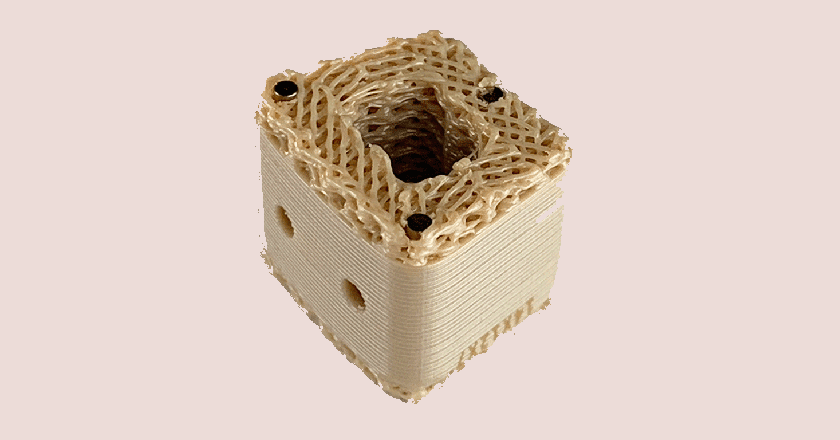
Inspire is reportedly the world’s first 3D-printed, fully interconnected porous PEEK structure, utilizing internally developed Fused Filament Fabrication.
The Inspire platform is manufactured with Evonik VESTAKEEP i4 3DF PEEK high-performance polymer on a proprietary, patented 3D printer designed, programmed and built by Curiteva.
This additive process produces a fully interconnected and integrated porous structure traversing the entire implant to promote osseointegration, improve radiographic assessment and deliver superior biomechanics. The combination of the HAFUSE nanotechnology surface treatment and novel porous PEEK structure creates a hydrophilic, bioactive environment for cell attachment, proliferation and healing in pre-clinical animal and in vitro studies.
“This new technology further demonstrates Curiteva’s commitment to developing and investing in disruptive technologies,” said Todd Reith, Inventor and Vice President of Emerging Technology. “As this technology matures, with our internal expertise, we have already contemplated other applications outside of spine and orthopedics.”
Source: Curiteva
Curiteva announced a limited commercial release utilizing its recently FDA-cleared Inspire Porous PEEK HAFUSE Cervical Interbody. Full commercial U.S. launch is slated for later in 2023.
Inspire is reportedly the world’s first 3D-printed, fully interconnected porous PEEK structure, utilizing internally developed Fused Filament...
Curiteva announced a limited commercial release utilizing its recently FDA-cleared Inspire Porous PEEK HAFUSE Cervical Interbody. Full commercial U.S. launch is slated for later in 2023.
Inspire is reportedly the world’s first 3D-printed, fully interconnected porous PEEK structure, utilizing internally developed Fused Filament Fabrication.
The Inspire platform is manufactured with Evonik VESTAKEEP i4 3DF PEEK high-performance polymer on a proprietary, patented 3D printer designed, programmed and built by Curiteva.
This additive process produces a fully interconnected and integrated porous structure traversing the entire implant to promote osseointegration, improve radiographic assessment and deliver superior biomechanics. The combination of the HAFUSE nanotechnology surface treatment and novel porous PEEK structure creates a hydrophilic, bioactive environment for cell attachment, proliferation and healing in pre-clinical animal and in vitro studies.
“This new technology further demonstrates Curiteva’s commitment to developing and investing in disruptive technologies,” said Todd Reith, Inventor and Vice President of Emerging Technology. “As this technology matures, with our internal expertise, we have already contemplated other applications outside of spine and orthopedics.”
Source: Curiteva


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.