
 Copy to clipboard
Copy to clipboard 
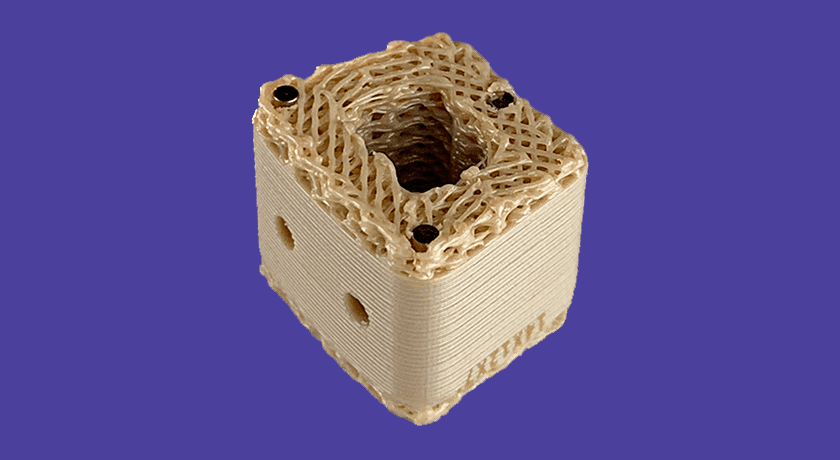
Curiteva announces what it states is the first FDA 510(k)-cleared 3D-printed PEEK implant, the Inspire Porous PEEK Cervical Interbody System with HAFUSE Technology. The company plans a commercial launch in key academic centers across the United States.
The Inspire platform is manufactured with a proprietary, patented Fused Filament Fabrication 3D printer designed, programmed and built by Curiteva. This additive process produces a fully interconnected and integrated porous structure traversing the entire implant to promote osseointegration, improve radiographic assessment and deliver superior biomechanics. The first-to-market combination of the HAFUSE nanotechnology surface treatment and novel porous PEEK structure creates a hydrophilic, bioactive environment for cell attachment, proliferation and healing in pre-clinical animal and in vitro studies.
“The distinctive Inspire implant technology enabled by our innovative 3D printing process incorporates an engineered lattice structure with fully interconnected porosity exhibiting superior mechanical strength and achieving a modulus of elasticity closely matching human cancellous bone,” said Co-Founder/Chief Technology Officer Eric Linder.
Source: Curiteva
Curiteva announces what it states is the first FDA 510(k)-cleared 3D-printed PEEK implant, the Inspire Porous PEEK Cervical Interbody System with HAFUSE Technology. The company plans a commercial launch in key academic centers across the United States.
The Inspire platform is manufactured with a proprietary, patented Fused Filament Fabrication...
Curiteva announces what it states is the first FDA 510(k)-cleared 3D-printed PEEK implant, the Inspire Porous PEEK Cervical Interbody System with HAFUSE Technology. The company plans a commercial launch in key academic centers across the United States.
The Inspire platform is manufactured with a proprietary, patented Fused Filament Fabrication 3D printer designed, programmed and built by Curiteva. This additive process produces a fully interconnected and integrated porous structure traversing the entire implant to promote osseointegration, improve radiographic assessment and deliver superior biomechanics. The first-to-market combination of the HAFUSE nanotechnology surface treatment and novel porous PEEK structure creates a hydrophilic, bioactive environment for cell attachment, proliferation and healing in pre-clinical animal and in vitro studies.
“The distinctive Inspire implant technology enabled by our innovative 3D printing process incorporates an engineered lattice structure with fully interconnected porosity exhibiting superior mechanical strength and achieving a modulus of elasticity closely matching human cancellous bone,” said Co-Founder/Chief Technology Officer Eric Linder.
Source: Curiteva

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







