
 Copy to clipboard
Copy to clipboard 
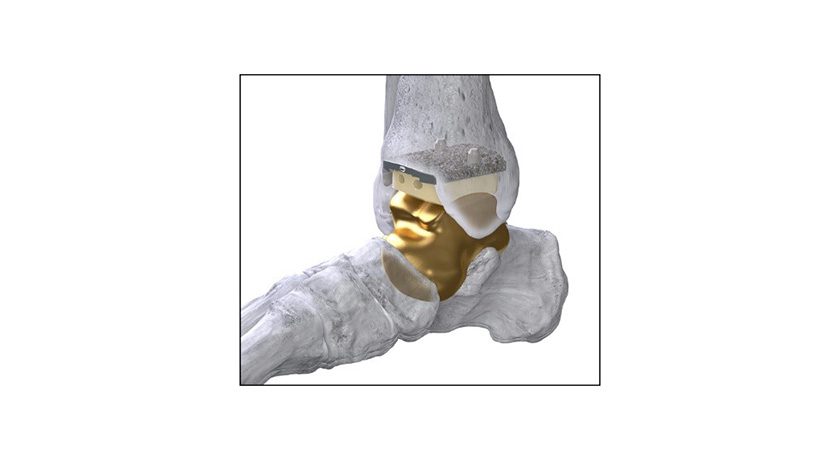
Paragon 28 received an Investigational Device Exemption (IDE) approval from FDA to commence a feasibility study for configurations of the SMART Total Talus System used in conjunction with the Paragon 28 APEX 3D Total Ankle Replacement. The study is expected to begin in early 2024.
Paragon 28 acquired Additive Orthopaedics in 2021, providing the company with the first and only FDA approved Patient Specific Total Talus replacement for treatment of avascular necrosis. With approval of the IDE to support future regulatory applications, the company’s SMART Total Talus is on the path to become the only device on the market intended for talar replacement in the setting of adjacent joint arthritis.
Source: Paragon 28, Inc.
Paragon 28 received an Investigational Device Exemption (IDE) approval from FDA to commence a feasibility study for configurations of the SMART Total Talus System used in conjunction with the Paragon 28 APEX 3D Total Ankle Replacement. The study is expected to begin in early 2024.
Paragon 28 acquired Additive Orthopaedics in 2021,...
Paragon 28 received an Investigational Device Exemption (IDE) approval from FDA to commence a feasibility study for configurations of the SMART Total Talus System used in conjunction with the Paragon 28 APEX 3D Total Ankle Replacement. The study is expected to begin in early 2024.
Paragon 28 acquired Additive Orthopaedics in 2021, providing the company with the first and only FDA approved Patient Specific Total Talus replacement for treatment of avascular necrosis. With approval of the IDE to support future regulatory applications, the company’s SMART Total Talus is on the path to become the only device on the market intended for talar replacement in the setting of adjacent joint arthritis.
Source: Paragon 28, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







