
 Copy to clipboard
Copy to clipboard 
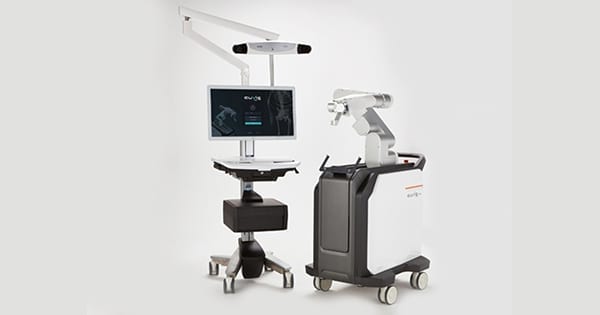
CUREXO was granted FDA 510(k) clearance to market the CUVIS-spine surgical robot.
The system is available globally, having secured approval under the CE Mark and regulatory clearance in Korea.
CUVIS-spine guides pedicle screw insertion according to the surgical plan, employing a high-precision robot arm and wireless one-step navigation based on a real-time OTS sensor to provide precise, safe and faster surgery vs. manual surgery. CUVIS-spine minimizes the filming and reduces the radiation exposure of both patients and medical staff.
Both 2-dimensional and 3-dimensional filming (C-arm and O-arm, respectively) are applicable with this solution, providing great expandability. Needle, K-wire, dilation and tapping of traditional manual surgery to be performed with one tool, reducing the length of a procedure.
Mr Jae-Joon Lee, CEO, said, “CUREXO plans to focus on our medical robot sales, including CUVIS-joint, CUVIS-spine and Morning Walk to not only the Korean market but also to advanced medical markets such as the U.S. and Europe.”
CUREXO was granted FDA 510(k) clearance to market the CUVIS-spine surgical robot.
The system is available globally, having secured approval under the CE Mark and regulatory clearance in Korea.
CUVIS-spine guides pedicle screw insertion according to the surgical plan, employing a high-precision robot arm and wireless one-step...
CUREXO was granted FDA 510(k) clearance to market the CUVIS-spine surgical robot.
The system is available globally, having secured approval under the CE Mark and regulatory clearance in Korea.
CUVIS-spine guides pedicle screw insertion according to the surgical plan, employing a high-precision robot arm and wireless one-step navigation based on a real-time OTS sensor to provide precise, safe and faster surgery vs. manual surgery. CUVIS-spine minimizes the filming and reduces the radiation exposure of both patients and medical staff.
Both 2-dimensional and 3-dimensional filming (C-arm and O-arm, respectively) are applicable with this solution, providing great expandability. Needle, K-wire, dilation and tapping of traditional manual surgery to be performed with one tool, reducing the length of a procedure.
Mr Jae-Joon Lee, CEO, said, “CUREXO plans to focus on our medical robot sales, including CUVIS-joint, CUVIS-spine and Morning Walk to not only the Korean market but also to advanced medical markets such as the U.S. and Europe.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







