
 Copy to clipboard
Copy to clipboard 
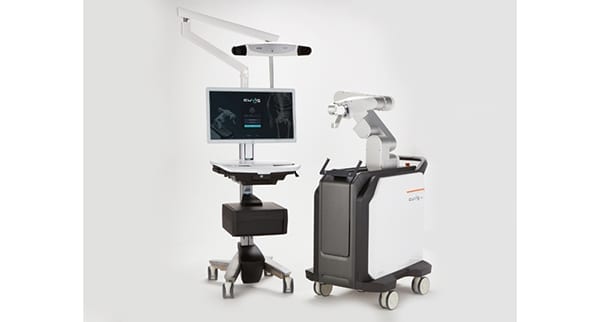
Curexo received approval under the CE Mark for the CUVIS-spine surgical robot. The company is also planning to apply for FDA clearance.
CUVIS-spine guides and supports the pedicle screw to the correct position using a robotic arm, one-step surgical tools and a real-time location sensor. Real-time patient position monitoring can correct the surgical plan in real time, and can help reduce radiation exposure for the patient and O.R. staff.
CUVIS-spine provides imaging compatibility on not only C-arms, but also O-arms. The device received approval by Korea’s Ministry of Food and Drug Safety and launched late last year in the region.
The company is also developing CUVIS-joint for potential launch in 2020.
According to the CEO of CUREXO, “Certifying the CE certificate for CUVIS-spine is another big step taken after last year’s MFDS certificate. Also, achieving the CE certificate for the CUVIS-spine is meaningful because now CUREXO is aiming for the global market with a self-developed surgical robot. The following task will be FDA approval and entering the U.S. market, which is number one in surgical robots.
Curexo received approval under the CE Mark for the CUVIS-spine surgical robot. The company is also planning to apply for FDA clearance.
CUVIS-spine guides and supports the pedicle screw to the correct position using a robotic arm, one-step surgical tools and a real-time location sensor. Real-time patient position monitoring can correct the...
Curexo received approval under the CE Mark for the CUVIS-spine surgical robot. The company is also planning to apply for FDA clearance.
CUVIS-spine guides and supports the pedicle screw to the correct position using a robotic arm, one-step surgical tools and a real-time location sensor. Real-time patient position monitoring can correct the surgical plan in real time, and can help reduce radiation exposure for the patient and O.R. staff.
CUVIS-spine provides imaging compatibility on not only C-arms, but also O-arms. The device received approval by Korea’s Ministry of Food and Drug Safety and launched late last year in the region.
The company is also developing CUVIS-joint for potential launch in 2020.
According to the CEO of CUREXO, “Certifying the CE certificate for CUVIS-spine is another big step taken after last year’s MFDS certificate. Also, achieving the CE certificate for the CUVIS-spine is meaningful because now CUREXO is aiming for the global market with a self-developed surgical robot. The following task will be FDA approval and entering the U.S. market, which is number one in surgical robots.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







