
 Copy to clipboard
Copy to clipboard 
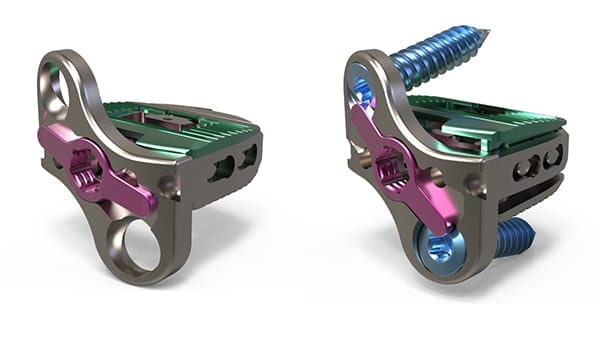
Atlas Spine received FDA 510(k) clearance to market the HiJAK SA Expandable Stand-Alone (SA) Cervical Interbody System.
HiJAK SA joins the company’s HiJAK AC and the V3 segmental plating in the company’s disruptive design portfolio. Both HiJAK systems allow intra-operative customization of height and lordosis. HiJAK SA incorporates an integrated low-profile plate that improves screw access during minimally invasive applications and screw-to-bone integrity, as an alternative to current stand-alone devices.
“It was our goal from the onset to develop the most comprehensive and innovative offerings for treating complex cervical pathologies,” said Matt Baynham, CEO and co-founder of Atlas Spine. “With the addition of the standalone variant to our expandable cervical interbody and guided segmental plating system, we can now address broader patient conditions and surgical preferences.”
Atlas Spine received FDA 510(k) clearance to market the HiJAK SA Expandable Stand-Alone (SA) Cervical Interbody System.
HiJAK SA joins the company's HiJAK AC and the V3 segmental plating in the company's disruptive design portfolio. Both HiJAK systems allow intra-operative customization of height and lordosis. HiJAK SA incorporates an...
Atlas Spine received FDA 510(k) clearance to market the HiJAK SA Expandable Stand-Alone (SA) Cervical Interbody System.
HiJAK SA joins the company’s HiJAK AC and the V3 segmental plating in the company’s disruptive design portfolio. Both HiJAK systems allow intra-operative customization of height and lordosis. HiJAK SA incorporates an integrated low-profile plate that improves screw access during minimally invasive applications and screw-to-bone integrity, as an alternative to current stand-alone devices.
“It was our goal from the onset to develop the most comprehensive and innovative offerings for treating complex cervical pathologies,” said Matt Baynham, CEO and co-founder of Atlas Spine. “With the addition of the standalone variant to our expandable cervical interbody and guided segmental plating system, we can now address broader patient conditions and surgical preferences.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







