
 Copy to clipboard
Copy to clipboard 
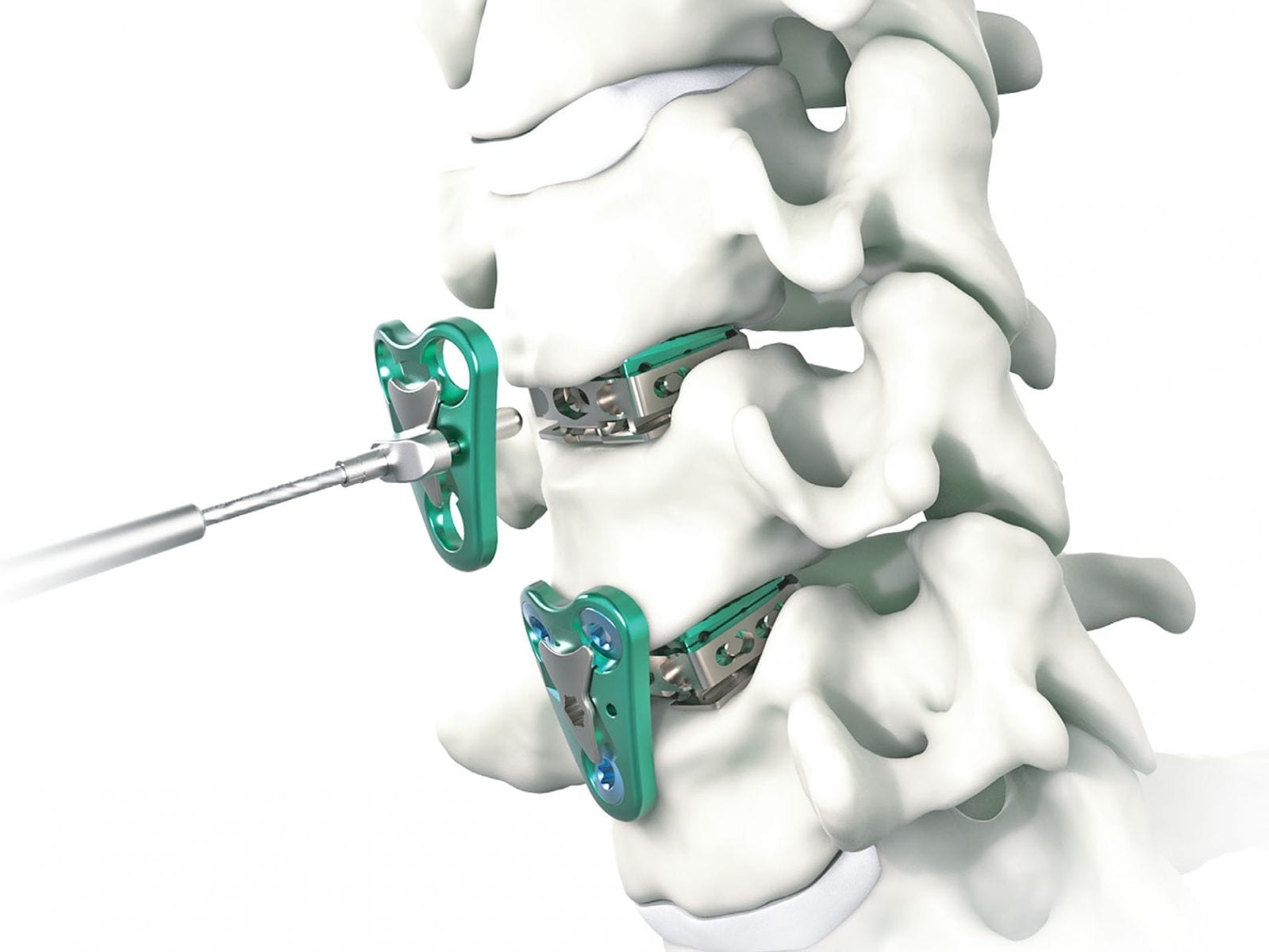
Atlas Spine launched the V3 Guided Segmental Plating System to treat complex deformity and degenerative conditions of the cervical spine.
The system works with the company’s HiJAK AC expandable cervical interbody. V3 guidance and its segmental approach address technical challenges during the plating process, providing a simple method to temporarily dock the place onto the face of HiJAK to allow control and confidence during installation. Compared to zero profile standalone devices, V3 offers increased rigidity by placing the plate on the anterior sideof the vertebral body. The plate allows an easier screw angulation, avoiding angled drivers and the risk of violating the endplate.
Studies indicate that a segmental plating method with its load sharing characteristics yields better outcomes for multi-level constructs vs. conventional plating.
This launch follows the 3Q19 FDA 510(k) clearance of the V3 system.
Atlas Spine launched the V3 Guided Segmental Plating System to treat complex deformity and degenerative conditions of the cervical spine.
The system works with the company's HiJAK AC expandable cervical interbody. V3 guidance and its segmental approach address technical challenges during the plating process, providing a simple method to...
Atlas Spine launched the V3 Guided Segmental Plating System to treat complex deformity and degenerative conditions of the cervical spine.
The system works with the company’s HiJAK AC expandable cervical interbody. V3 guidance and its segmental approach address technical challenges during the plating process, providing a simple method to temporarily dock the place onto the face of HiJAK to allow control and confidence during installation. Compared to zero profile standalone devices, V3 offers increased rigidity by placing the plate on the anterior sideof the vertebral body. The plate allows an easier screw angulation, avoiding angled drivers and the risk of violating the endplate.
Studies indicate that a segmental plating method with its load sharing characteristics yields better outcomes for multi-level constructs vs. conventional plating.
This launch follows the 3Q19 FDA 510(k) clearance of the V3 system.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







