
 Copy to clipboard
Copy to clipboard 
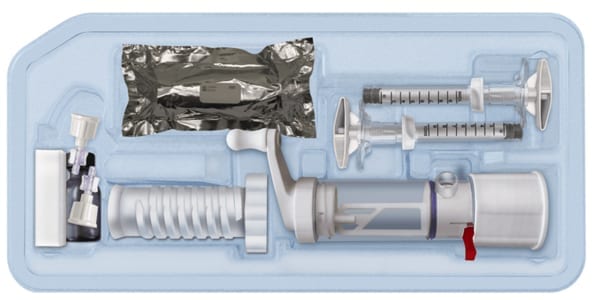
AgNovos Healthcare’s investigational product, AGN1 Local Osteo-enhancement Procedure (LOEP) Small Volume (SV) kit, received an Investigational Device Exemption from FDA. The IDE will support the study of the device’s ability to reduce pain and support mobility in patients with vertebral compression fractures.
AGN1 LOEP SV, which was granted Breakthrough Device designation by FDA last year, is an alternative to traditional vertebral augmentation and is intended to treat stable but painful vertebral compression fractures in a minimally invasive procedure.
The kit contains all instruments and components to prepare the fractured vertebral body for injection with AGN1, a resorbable, osteoconductive, tri-phasic implant material. The AGN1 implant material is available in a CE Marked kit to form new bone in the pelvis and other extremities.
Preclinical and clinical research has shown that treatment with the kit can lead to the formation of new bone and an immediate, substantial and durable increase in the strength of osteoporotic femurs.
Tanner Howe, President and CEO of AgNovos Healthcare, said, “We believe that the AGN1 LOEP SV kit has the potential to transform the treatment landscape for vertebral compression fractures. It is designed to work differently than traditional vertebral augmentation because the AGN1 implant material is intended to be resorbed and replaced by new bone. This treatment is in line with the Company’s mission of improving the quality of life of millions of women and men impacted every day by bone disease, including osteoporosis.”
AgNovos Healthcare's investigational product, AGN1 Local Osteo-enhancement Procedure (LOEP) Small Volume (SV) kit, received an Investigational Device Exemption from FDA. The IDE will support the study of the device’s ability to reduce pain and support mobility in patients with vertebral compression fractures.
AGN1 LOEP SV, which was...
AgNovos Healthcare’s investigational product, AGN1 Local Osteo-enhancement Procedure (LOEP) Small Volume (SV) kit, received an Investigational Device Exemption from FDA. The IDE will support the study of the device’s ability to reduce pain and support mobility in patients with vertebral compression fractures.
AGN1 LOEP SV, which was granted Breakthrough Device designation by FDA last year, is an alternative to traditional vertebral augmentation and is intended to treat stable but painful vertebral compression fractures in a minimally invasive procedure.
The kit contains all instruments and components to prepare the fractured vertebral body for injection with AGN1, a resorbable, osteoconductive, tri-phasic implant material. The AGN1 implant material is available in a CE Marked kit to form new bone in the pelvis and other extremities.
Preclinical and clinical research has shown that treatment with the kit can lead to the formation of new bone and an immediate, substantial and durable increase in the strength of osteoporotic femurs.
Tanner Howe, President and CEO of AgNovos Healthcare, said, “We believe that the AGN1 LOEP SV kit has the potential to transform the treatment landscape for vertebral compression fractures. It is designed to work differently than traditional vertebral augmentation because the AGN1 implant material is intended to be resorbed and replaced by new bone. This treatment is in line with the Company’s mission of improving the quality of life of millions of women and men impacted every day by bone disease, including osteoporosis.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







