
 Copy to clipboard
Copy to clipboard 
AgNovos Healthcare was granted Breakthrough Device Designation by FDA for the AGN1 Local Osteo-enhancement Procedure Small Volume (LOEP SV) Kit. If cleared, the kit would represent a breakthrough treatment for stable vertebral compression fractures.
AGN1 LOEP SV contains a proprietary calcium-based resorbable tri-phasic implant material formulated to couple the pace of resorption to bone formation, allowing treated vertebrae immediate, substantial protection. A kit is already approved in Europe for larger volume applications at other anatomical sites.
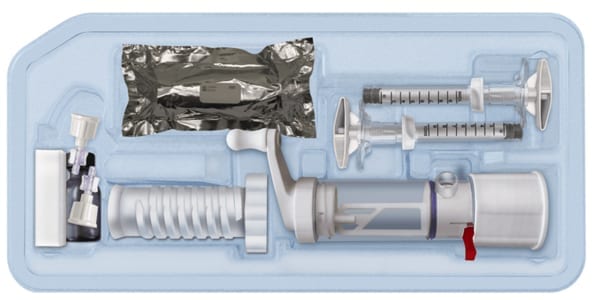
The AGN1 LOEP SV Kit is an investigational device intended to minimally-invasively treat painful but stable vertebral compression fractures that are often caused by bone loss associated with osteoporosis. The kit contains the instruments and components necessary to prepare the fractured vertebral body for injection.
“Our receipt of the Breakthrough Device Designation is a welcome milestone in realizing our mission of saving lives and protecting the quality of life of patients,” said Tanner Howe, President and CEO. “We believe that the device would give clinicians a powerful new tool to treat stable vertebral compression fractures in a more biologically-congruent fashion than they are treated today.”
FDA’s Breakthrough Devices Program is intended to expedite the development and review of devices that are either novel or have the potential to benefit patients with life-threatening or debilitating conditions. By accelerating the process of development and review, it is hoped that clinicians and patients will have faster access to devices that can improve the quality of care.
AgNovos Healthcare was granted Breakthrough Device Designation by FDA for the AGN1 Local Osteo-enhancement Procedure Small Volume (LOEP SV) Kit. If cleared, the kit would represent a breakthrough treatment for stable vertebral compression fractures.
AGN1 LOEP SV contains a proprietary calcium-based resorbable tri-phasic implant material...
AgNovos Healthcare was granted Breakthrough Device Designation by FDA for the AGN1 Local Osteo-enhancement Procedure Small Volume (LOEP SV) Kit. If cleared, the kit would represent a breakthrough treatment for stable vertebral compression fractures.
AGN1 LOEP SV contains a proprietary calcium-based resorbable tri-phasic implant material formulated to couple the pace of resorption to bone formation, allowing treated vertebrae immediate, substantial protection. A kit is already approved in Europe for larger volume applications at other anatomical sites.
The AGN1 LOEP SV Kit is an investigational device intended to minimally-invasively treat painful but stable vertebral compression fractures that are often caused by bone loss associated with osteoporosis. The kit contains the instruments and components necessary to prepare the fractured vertebral body for injection.
“Our receipt of the Breakthrough Device Designation is a welcome milestone in realizing our mission of saving lives and protecting the quality of life of patients,” said Tanner Howe, President and CEO. “We believe that the device would give clinicians a powerful new tool to treat stable vertebral compression fractures in a more biologically-congruent fashion than they are treated today.”
FDA’s Breakthrough Devices Program is intended to expedite the development and review of devices that are either novel or have the potential to benefit patients with life-threatening or debilitating conditions. By accelerating the process of development and review, it is hoped that clinicians and patients will have faster access to devices that can improve the quality of care.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







