
 Copy to clipboard
Copy to clipboard 
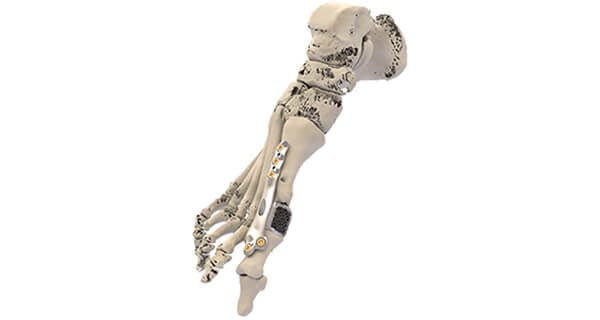
Additive Orthopaedics announced their ability to incorporate locking technology in all of their Patient Specific Implants for trauma applications. The patent-pending technology can be incorporated in the company’s Patient Specific 3D-Printed Bone Segments and Patient Specific 3D-Printed Plates, pictured above.
Additive Orthopaedics is the only company to have FDA 510(k) clearances for Patient Specific 3D-Printed Bone Segments and Patient Specific 3D-Printed Plates for use in the foot and ankle. The company is developing Patient Specific Implants leveraging Game Plan®, an end-to-end fully integrated cloud-based communication system that aligns surgeons and engineers to optimize patient outcomes.
Luciano Bertolotti, Director of Engineering for Additive Orthopaedics, said, “Locking technology is straightforward for off the shelf implants in which the machining process for the screw hole threads is set up once and can be manufactured in high volumes. Conversely, the geometries of Patient Specific Implants can be highly complex. Every Patient Specific Implant is unique and each case poses a new challenge. Regardless of the complexity, we are now able to incorporate locking threads into the contour of the implant and deliver in a quick timeline. The ability to offer locking technology in our Patient Specific Implants is another way Additive is continuing to innovate and provide better solutions for surgeons and their patients.”
Additive Orthopaedics announced their ability to incorporate locking technology in all of their Patient Specific Implants for trauma applications. The patent-pending technology can be incorporated in the company's Patient Specific 3D-Printed Bone Segments and Patient Specific 3D-Printed Plates, pictured above.
Additive Orthopaedics is...
Additive Orthopaedics announced their ability to incorporate locking technology in all of their Patient Specific Implants for trauma applications. The patent-pending technology can be incorporated in the company’s Patient Specific 3D-Printed Bone Segments and Patient Specific 3D-Printed Plates, pictured above.
Additive Orthopaedics is the only company to have FDA 510(k) clearances for Patient Specific 3D-Printed Bone Segments and Patient Specific 3D-Printed Plates for use in the foot and ankle. The company is developing Patient Specific Implants leveraging Game Plan®, an end-to-end fully integrated cloud-based communication system that aligns surgeons and engineers to optimize patient outcomes.
Luciano Bertolotti, Director of Engineering for Additive Orthopaedics, said, “Locking technology is straightforward for off the shelf implants in which the machining process for the screw hole threads is set up once and can be manufactured in high volumes. Conversely, the geometries of Patient Specific Implants can be highly complex. Every Patient Specific Implant is unique and each case poses a new challenge. Regardless of the complexity, we are now able to incorporate locking threads into the contour of the implant and deliver in a quick timeline. The ability to offer locking technology in our Patient Specific Implants is another way Additive is continuing to innovate and provide better solutions for surgeons and their patients.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.