Stay up to date on the orthopedic market’s 3Q performance in 2025 as we update this post with company sales data and industry developments.
November 10, 2025
,
Mike Evers
The Numbers
The company grew in the high single digits on a reported basis for the third quarter of 2025 and saw improved U.S. performance.
Excluding its divested spinal implant business, Stryker’s orthopedic sales grew in the double digits for the third quarter of 2025.
Orthoflash®
The implant design reinforces the damaged labral region with a smooth, cartilage-friendly articulating surface engineered from the company’s BioPoly material.
November 7, 2025
,
Julie A. Vetalice
The PHANTOM series joins Aevumed’s portfolio of fixation solutions designed to improve patient outcomes while supporting surgeon efficiency in the operating room.
November 7, 2025
,
Julie A. Vetalice
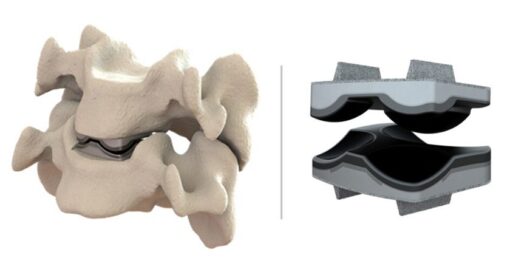
The UniSpace lumbar device was designed to be compatible with CGBio’s next-generation NOVOSIS Putty bone graft substitute.
November 7, 2025
,
Julie A. Vetalice
The system is designed to provide truly patient-specific custom solutions for revisions, deformities, arthritic conditions, and trauma-related foot and ankle injuries.
November 7, 2025
,
Julie A. Vetalice
The synthetic, absorbable gel is for use as an adjunct in lumbar spine surgery for the reduction of leg pain and neurological symptoms.
November 5, 2025
,
Patrick McGuire
Competitive Landscape
Industry Voices