
 Copy to clipboard
Copy to clipboard 
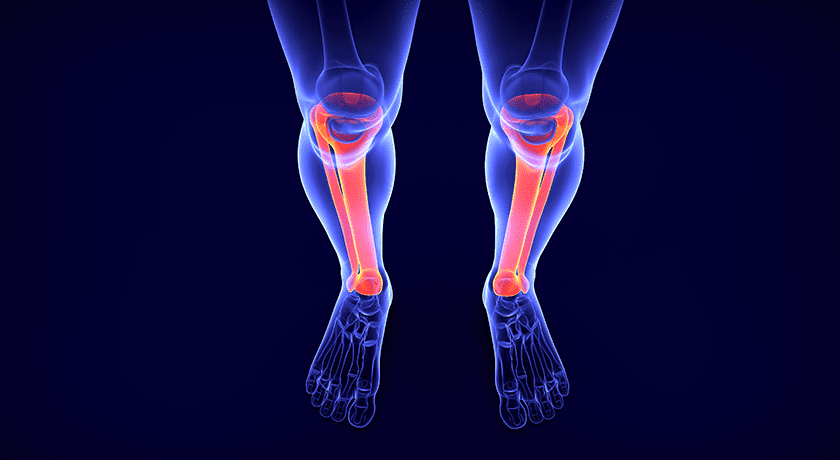
Meduloc announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its proprietary intramedullary fracture fixation system. This clearance marks a significant milestone as Meduloc introduces a new category of fixation designed to be able to treat small, long bone fractures.
The Meduloc system offers surgeons a differentiated, approach to fracture fixation, combining a strong, flexible nitinol implant with a deployable prong locking mechanism. The design delivers rotational and length stability while enabling surgeons the choice of avoiding entry through the joint capsule — a technique which the company says may enable earlier mobility, fewer complications, reduced surgical complexity, and allow for faster patient recovery. The platform addresses multiple indications, including metacarpal, radius, ulna, clavicle, and fibula fractures, for both adult and pediatric populations.
With clearance secured, Meduloc is preparing for a targeted U.S. commercial launch and expanding its network of surgeon advisors and clinical partners to support adoption and generate real-world evidence.
“An orthopedic surgeon came to us with a problem, and we developed a solution,” said Sarah Sachinis, CEO and President of Meduloc. “We’re excited to bring this innovation to market and empower surgeons with technology that improves their ability to treat patients with small bone fractures more effectively.”
Source: Meduloc
Meduloc announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its proprietary intramedullary fracture fixation system. This clearance marks a significant milestone as Meduloc introduces a new category of fixation designed to be able to treat small, long bone fractures.
The Meduloc system offers surgeons a...
Meduloc announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its proprietary intramedullary fracture fixation system. This clearance marks a significant milestone as Meduloc introduces a new category of fixation designed to be able to treat small, long bone fractures.
The Meduloc system offers surgeons a differentiated, approach to fracture fixation, combining a strong, flexible nitinol implant with a deployable prong locking mechanism. The design delivers rotational and length stability while enabling surgeons the choice of avoiding entry through the joint capsule — a technique which the company says may enable earlier mobility, fewer complications, reduced surgical complexity, and allow for faster patient recovery. The platform addresses multiple indications, including metacarpal, radius, ulna, clavicle, and fibula fractures, for both adult and pediatric populations.
With clearance secured, Meduloc is preparing for a targeted U.S. commercial launch and expanding its network of surgeon advisors and clinical partners to support adoption and generate real-world evidence.
“An orthopedic surgeon came to us with a problem, and we developed a solution,” said Sarah Sachinis, CEO and President of Meduloc. “We’re excited to bring this innovation to market and empower surgeons with technology that improves their ability to treat patients with small bone fractures more effectively.”
Source: Meduloc

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
PM
Patrick McGuire is an ORTHOWORLD Contributor.