Copy to clipboard
Copy to clipboard 
BioPoly recently received an Innovative Technology contract from Vizient, signaling the company’s synthetic cartilage implants’ potential to improve orthopedic outcomes. The news is the latest in a string of announcements from the company, which seeks to provide early intervention cartilage repair solutions that bridge the gap between biological treatments and total joint replacement.
BioPoly’s platform material is used to manufacture implants throughout the body, including the toes, radial head, shoulder and knee, and numerous other indications are in the product pipeline. The company sells in Europe, parts of Southeast Asia and Canada, and more recently entered the U.S. market with its Greater Toe, Lesser Toe and Radial Head implants.
Material Differentiation
BioPoly’s implants are engineered from a microcomposite material that integrates ultra-high molecular weight polyethylene (UHMWPE) with hyaluronic acid (HA). Company Founder, President and CEO Herb Schwartz, Ph.D., said they licensed the biomaterial from Colorado State University and spent years developing it so that it could emulate the low-friction, load-bearing characteristics of native cartilage.
HA helps provide a frictionless surface in a synovial joint, while UHMWPE allows the implant to carry significant anatomical loads. BioPoly’s devices interact synergistically with the surrounding cartilage surfaces, supporting natural biomechanics and promoting joint preservation.
Dr. Schwartz said a unique characteristic of the material is the large difference in the coefficient of friction between a metal implant articulating on cartilage and BioPoly on cartilage. BioPoly on cartilage has an 85% lower coefficient of friction than cobalt chrome and 40% less cartilage wear than cobalt chrome when articulating against cartilage.
“It took us a long time to get the properties to meet ASTM standards,” Dr. Schwartz said, “but in doing so, we developed a lot of trade secrets to manufacture the material so that we can sustain standard loads.”
Targeting the Knee Market
Studies have shown that more than 60% of patients undergoing total knee replacement have cartilage defects. These results mean that there is a sizable need for early-stage interventions.
BioPoly began commercializing its partial resurfacing knee implant in Europe in 2012 after receiving CE Mark certification. Two clinical studies published in the Journal of Bone and Joint Surgery demonstrated its success. A five-year study found that the implant was safe, provided significant improvement starting at six months and continuing to five years, and provided greater improvement than microfracture for some outcome measures.
The BioPoly knee received FDA Breakthrough Device Designation in 2023 for the indication for partial resurfacing in the femoral condyle or trochlear facet. In April 2025, the company commenced patient recruitment for its IDE study, which focuses on cartilage defects of the distal femur of the knee.
“Listing our IDE clinical study and patient recruitment through ClinicalTrials.gov underscores BioPoly’s commitment to transparency, scientific rigor and patient care,” Dr. Schwartz said at the time of the announcement. “With our first site up and running, this milestone brings us closer to providing a novel, less invasive treatment option that preserves the natural anatomy of the knee and significantly enhances patient outcomes.”
The company commercializes its patella and trochlea implants outside the U.S. and plans to submit its FDA 510(k) submission for the patella in the coming months. Further, BioPoly is developing a patellofemoral joint replacement.
“Pain under the kneecap is very difficult to treat,” Dr. Schwartz said. “Most patellofemoral joint implants resurface the patella with polyethylene. However, polyethylene and cartilage don’t do well against each other, so they resurface the trochlear groove with a metal implant. We can replace just the damaged area with metal and place BioPoly on the other side for better articulation.”
Making Inroads in the Extremities Market
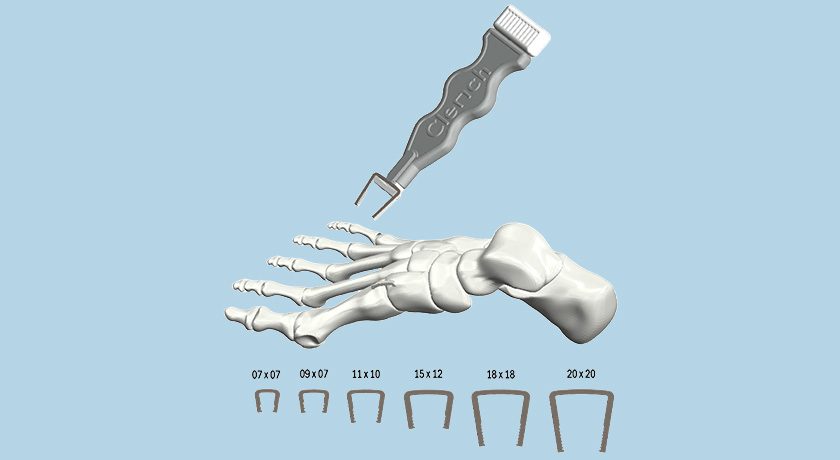
BioPoly also has a robust pipeline for extremity applications. The company first entered the U.S. market with its Great Toe System and Lesser Toe System, which received FDA 510(k) clearance in 2021 and 2022, respectively. It also received clearance for its Radial Head Implant and performed the first surgery in February of this year.
“The radial head clearance is particularly exciting for us, because we address two of the main failure modes: loosening and cartilage erosion,” Dr. Schwartz said. “There are two philosophies for radial head design: a fixed stem or a loose stem, spacer design. We use a spacer design, which is easier for the surgeon to implant. The device also finds its center on its own, reducing stress on the implant and the joint.”
In March, BioPoly also announced a U.S. patent for its Hill-Sachs Implant to partially resurface the shoulder joint. The implant is designed to stabilize the shoulder joint in patients who experience chronic dislocation.
Dr. Schwartz said the company’s primary goals in the U.S. market in 2025 are to drive sales of its toe implants and radial head implant, while continuing to balance R&D and regulatory pathways for future products. In extremities alone, the company’s pipeline includes the thumb, finger, wrist and ankle.
New Contract Sets Stage for Growth
One of the biggest challenges small companies face today is getting approval from value analysis committees to contract with hospitals. Navigating individual hospital systems’ timelines and processes requires connections, resources and skills.
BioPoly’s new Innovative Technology contract with Vizient gives hospitals across the U.S. access to the company’s technology. Vizient’s client base of hospitals, academic medical systems and integrated health delivery networks represents more than $140 billion in annual purchasing volume.
Innovative Technology contracts are granted after review and interaction with products submitted through Vizient’s Innovative Technology Program. Vizient client-led councils identify technologies that have the potential to enhance clinical care and agreed that BioPoly offers a unique and incremental benefit over other products available on the market.
The contract validates BioPoly’s work over the last two decades and could provide the company with momentum as it rolls out its proprietary material across additional indications. Dr. Schwartz said that the company is even working on a Vitamin E BioPoly for total joints that could extend the life of the joint in younger patients.
“We’re excited about where the company is today,” Dr. Schwartz said. “I’ve been at this for a long time, and it’s exciting to see that we’re helping many patients in a lot of different areas.”
BioPoly recently received an Innovative Technology contract from Vizient, signaling the company’s synthetic cartilage implants’ potential to improve orthopedic outcomes. The news is the latest in a string of announcements from the company, which seeks to provide early intervention cartilage repair solutions that bridge the gap between biological...
BioPoly recently received an Innovative Technology contract from Vizient, signaling the company’s synthetic cartilage implants’ potential to improve orthopedic outcomes. The news is the latest in a string of announcements from the company, which seeks to provide early intervention cartilage repair solutions that bridge the gap between biological treatments and total joint replacement.
BioPoly’s platform material is used to manufacture implants throughout the body, including the toes, radial head, shoulder and knee, and numerous other indications are in the product pipeline. The company sells in Europe, parts of Southeast Asia and Canada, and more recently entered the U.S. market with its Greater Toe, Lesser Toe and Radial Head implants.
Material Differentiation
BioPoly’s implants are engineered from a microcomposite material that integrates ultra-high molecular weight polyethylene (UHMWPE) with hyaluronic acid (HA). Company Founder, President and CEO Herb Schwartz, Ph.D., said they licensed the biomaterial from Colorado State University and spent years developing it so that it could emulate the low-friction, load-bearing characteristics of native cartilage.
HA helps provide a frictionless surface in a synovial joint, while UHMWPE allows the implant to carry significant anatomical loads. BioPoly’s devices interact synergistically with the surrounding cartilage surfaces, supporting natural biomechanics and promoting joint preservation.
Dr. Schwartz said a unique characteristic of the material is the large difference in the coefficient of friction between a metal implant articulating on cartilage and BioPoly on cartilage. BioPoly on cartilage has an 85% lower coefficient of friction than cobalt chrome and 40% less cartilage wear than cobalt chrome when articulating against cartilage.
“It took us a long time to get the properties to meet ASTM standards,” Dr. Schwartz said, “but in doing so, we developed a lot of trade secrets to manufacture the material so that we can sustain standard loads.”
Targeting the Knee Market
Studies have shown that more than 60% of patients undergoing total knee replacement have cartilage defects. These results mean that there is a sizable need for early-stage interventions.
BioPoly began commercializing its partial resurfacing knee implant in Europe in 2012 after receiving CE Mark certification. Two clinical studies published in the Journal of Bone and Joint Surgery demonstrated its success. A five-year study found that the implant was safe, provided significant improvement starting at six months and continuing to five years, and provided greater improvement than microfracture for some outcome measures.
The BioPoly knee received FDA Breakthrough Device Designation in 2023 for the indication for partial resurfacing in the femoral condyle or trochlear facet. In April 2025, the company commenced patient recruitment for its IDE study, which focuses on cartilage defects of the distal femur of the knee.
“Listing our IDE clinical study and patient recruitment through ClinicalTrials.gov underscores BioPoly’s commitment to transparency, scientific rigor and patient care,” Dr. Schwartz said at the time of the announcement. “With our first site up and running, this milestone brings us closer to providing a novel, less invasive treatment option that preserves the natural anatomy of the knee and significantly enhances patient outcomes.”
The company commercializes its patella and trochlea implants outside the U.S. and plans to submit its FDA 510(k) submission for the patella in the coming months. Further, BioPoly is developing a patellofemoral joint replacement.
“Pain under the kneecap is very difficult to treat,” Dr. Schwartz said. “Most patellofemoral joint implants resurface the patella with polyethylene. However, polyethylene and cartilage don’t do well against each other, so they resurface the trochlear groove with a metal implant. We can replace just the damaged area with metal and place BioPoly on the other side for better articulation.”
Making Inroads in the Extremities Market
BioPoly also has a robust pipeline for extremity applications. The company first entered the U.S. market with its Great Toe System and Lesser Toe System, which received FDA 510(k) clearance in 2021 and 2022, respectively. It also received clearance for its Radial Head Implant and performed the first surgery in February of this year.
“The radial head clearance is particularly exciting for us, because we address two of the main failure modes: loosening and cartilage erosion,” Dr. Schwartz said. “There are two philosophies for radial head design: a fixed stem or a loose stem, spacer design. We use a spacer design, which is easier for the surgeon to implant. The device also finds its center on its own, reducing stress on the implant and the joint.”
In March, BioPoly also announced a U.S. patent for its Hill-Sachs Implant to partially resurface the shoulder joint. The implant is designed to stabilize the shoulder joint in patients who experience chronic dislocation.
Dr. Schwartz said the company’s primary goals in the U.S. market in 2025 are to drive sales of its toe implants and radial head implant, while continuing to balance R&D and regulatory pathways for future products. In extremities alone, the company’s pipeline includes the thumb, finger, wrist and ankle.
New Contract Sets Stage for Growth
One of the biggest challenges small companies face today is getting approval from value analysis committees to contract with hospitals. Navigating individual hospital systems’ timelines and processes requires connections, resources and skills.
BioPoly’s new Innovative Technology contract with Vizient gives hospitals across the U.S. access to the company’s technology. Vizient’s client base of hospitals, academic medical systems and integrated health delivery networks represents more than $140 billion in annual purchasing volume.
Innovative Technology contracts are granted after review and interaction with products submitted through Vizient’s Innovative Technology Program. Vizient client-led councils identify technologies that have the potential to enhance clinical care and agreed that BioPoly offers a unique and incremental benefit over other products available on the market.
The contract validates BioPoly’s work over the last two decades and could provide the company with momentum as it rolls out its proprietary material across additional indications. Dr. Schwartz said that the company is even working on a Vitamin E BioPoly for total joints that could extend the life of the joint in younger patients.
“We’re excited about where the company is today,” Dr. Schwartz said. “I’ve been at this for a long time, and it’s exciting to see that we’re helping many patients in a lot of different areas.”

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
CL
Carolyn LaWell is ORTHOWORLD's Chief Content Officer. She joined ORTHOWORLD in 2012 to oversee its editorial and industry education. She previously served in editor roles at B2B magazines and newspapers.