
 Copy to clipboard
Copy to clipboard 
ZygoFix was granted approval under the CE Mark for its minimally invasive screwless spinal fusion system for the treatment of chronic back pain, the zLOCK Spinal Fusion System.
ZygoFix completed the certification process and received CE Mark for its zLOCK Spinal Fusion System for lumbar spine after demonstrating its safety and efficacy in clinical studies. Together with this certification, ZygoFix also received ISO13485 certification.
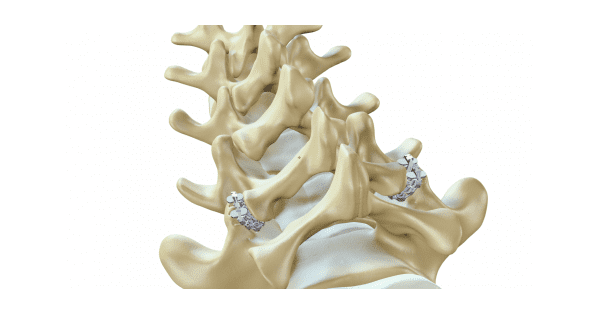
The zLOCK system’s miniature 3D-printed implant utilizes the spine’s anatomy for spinal stability, rather than traditional external screw-based stabilization. Its unique features combine rigid and bendable elements that enable the implant to withstand loads applied through the spine, while conforming to each patient’s anatomy during implantation. The procedure eliminates risks associated with improperly placed screws, is less invasive (2 versus 6 incisions), simple to use, and suitable for outpatient implantation.
The zLOCK system has been in clinical use for more than three years in Hungary and Israel with high levels of patient and physician satisfaction.
ZygoFix CEO Ofer Levy remarks: “Receiving CE Mark is a key event for the zLOCK system as a minimally invasive and safer solution for back pain sufferers. ZygoFix is opening a multi-center pivotal study in the EU to collect further clinical data.”
Source: ZygoFix
ZygoFix was granted approval under the CE Mark for its minimally invasive screwless spinal fusion system for the treatment of chronic back pain, the zLOCK Spinal Fusion System.
ZygoFix completed the certification process and received CE Mark for its zLOCK Spinal Fusion System for lumbar spine after demonstrating its safety and efficacy in...
ZygoFix was granted approval under the CE Mark for its minimally invasive screwless spinal fusion system for the treatment of chronic back pain, the zLOCK Spinal Fusion System.
ZygoFix completed the certification process and received CE Mark for its zLOCK Spinal Fusion System for lumbar spine after demonstrating its safety and efficacy in clinical studies. Together with this certification, ZygoFix also received ISO13485 certification.
The zLOCK system’s miniature 3D-printed implant utilizes the spine’s anatomy for spinal stability, rather than traditional external screw-based stabilization. Its unique features combine rigid and bendable elements that enable the implant to withstand loads applied through the spine, while conforming to each patient’s anatomy during implantation. The procedure eliminates risks associated with improperly placed screws, is less invasive (2 versus 6 incisions), simple to use, and suitable for outpatient implantation.
The zLOCK system has been in clinical use for more than three years in Hungary and Israel with high levels of patient and physician satisfaction.
ZygoFix CEO Ofer Levy remarks: “Receiving CE Mark is a key event for the zLOCK system as a minimally invasive and safer solution for back pain sufferers. ZygoFix is opening a multi-center pivotal study in the EU to collect further clinical data.”
Source: ZygoFix

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







