
 Copy to clipboard
Copy to clipboard 
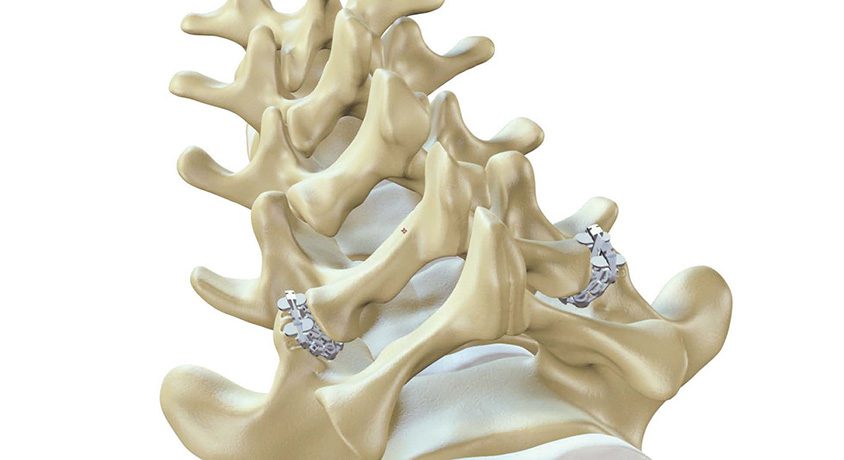
ZygoFix received FDA 510(k) clearance to market its zLOCK Lumbar Facet Fixation System. This achievement was supported by compelling clinical evidence from the company’s ongoing European clinical study, marking a significant advancement in spinal fusion technology.
As an alternative to the traditional placement of screws and rods to create an artificial bridge, stabilizing the segment, the zLOCK system harnesses the spine’s innate bone structure to securely lock its connecting joints. Unlike traditional methods, the company’s implant adapts to the joint’s anatomy during insertion, offering stability with a minimally invasive procedure that can be performed in outpatient and ASC environments.
ZygoFix CEO Ofer Levy remarked, “Receiving FDA clearance for our zLOCK Lumbar Stabilization System is an exciting milestone, and I am very proud of the team that has worked hard to achieve this goal. The system has garnered significant interest in the US market and this clearance paves the way for us to introduce our transformative solution to the US market, fostering collaboration with leading surgeons and enhancing patient care.”
Source: ZygoFix
ZygoFix received FDA 510(k) clearance to market its zLOCK Lumbar Facet Fixation System. This achievement was supported by compelling clinical evidence from the company’s ongoing European clinical study, marking a significant advancement in spinal fusion technology.
As an alternative to the traditional placement of screws and rods to create an...
ZygoFix received FDA 510(k) clearance to market its zLOCK Lumbar Facet Fixation System. This achievement was supported by compelling clinical evidence from the company’s ongoing European clinical study, marking a significant advancement in spinal fusion technology.
As an alternative to the traditional placement of screws and rods to create an artificial bridge, stabilizing the segment, the zLOCK system harnesses the spine’s innate bone structure to securely lock its connecting joints. Unlike traditional methods, the company’s implant adapts to the joint’s anatomy during insertion, offering stability with a minimally invasive procedure that can be performed in outpatient and ASC environments.
ZygoFix CEO Ofer Levy remarked, “Receiving FDA clearance for our zLOCK Lumbar Stabilization System is an exciting milestone, and I am very proud of the team that has worked hard to achieve this goal. The system has garnered significant interest in the US market and this clearance paves the way for us to introduce our transformative solution to the US market, fostering collaboration with leading surgeons and enhancing patient care.”
Source: ZygoFix

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







