
 Copy to clipboard
Copy to clipboard 
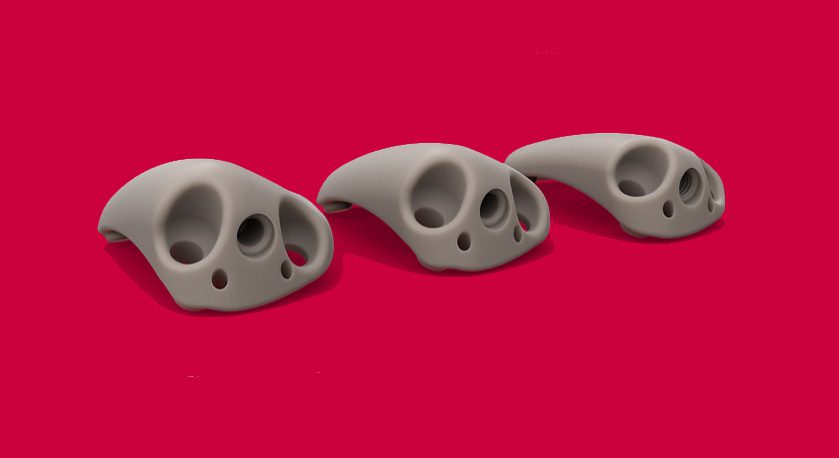
ZKR Orthopedics announced the successful performance of the first U.S. patella LIFT procedure, initiating its FDA PELICAN trial.
Patella LIFT offers a minimally invasive method to unload the patella and relieve pain without the complications associated with other treatments. Patients are allowed immediate range of motion and weight bearing, allowing faster return to activities. As the procedure is less invasive and less expensive than existing options, it may be attractive to ASCs and Payors.
ZKR received FDA approval in February 2024 for its IDE trial based on a successful European Pilot study. That 2-year study on 18 patients demonstrated significant improvement in pain and function, particularly in stair climbing, squatting and walking. Patients were able to successfully resume sports after the ZKR implant including tennis, mountain biking, skiing and hiking.
The PELICAN study will evaluate patients with later stage cartilage degeneration and osteoarthritis of the patellofemoral joint. The study will compare the results of the ZKR Patella LIFT Implant performed at centers in the United States to a control group consisting of tibial tubercle osteotomy procedures performed at various centers in Europe. The trial’s primary endpoints comprise patient-reported outcomes, safety measures and radiographic confirmation. Pain, function and speed of recovery will make up the secondary endpoints.
The PELICAN trial with ultimately include a total of 10 investigational sites in the U.S. and 10 in Europe.
Source: ZKR Orthopedics
ZKR Orthopedics announced the successful performance of the first U.S. patella LIFT procedure, initiating its FDA PELICAN trial.
Patella LIFT offers a minimally invasive method to unload the patella and relieve pain without the complications associated with other treatments. Patients are allowed immediate range of motion and weight bearing,...
ZKR Orthopedics announced the successful performance of the first U.S. patella LIFT procedure, initiating its FDA PELICAN trial.
Patella LIFT offers a minimally invasive method to unload the patella and relieve pain without the complications associated with other treatments. Patients are allowed immediate range of motion and weight bearing, allowing faster return to activities. As the procedure is less invasive and less expensive than existing options, it may be attractive to ASCs and Payors.
ZKR received FDA approval in February 2024 for its IDE trial based on a successful European Pilot study. That 2-year study on 18 patients demonstrated significant improvement in pain and function, particularly in stair climbing, squatting and walking. Patients were able to successfully resume sports after the ZKR implant including tennis, mountain biking, skiing and hiking.
The PELICAN study will evaluate patients with later stage cartilage degeneration and osteoarthritis of the patellofemoral joint. The study will compare the results of the ZKR Patella LIFT Implant performed at centers in the United States to a control group consisting of tibial tubercle osteotomy procedures performed at various centers in Europe. The trial’s primary endpoints comprise patient-reported outcomes, safety measures and radiographic confirmation. Pain, function and speed of recovery will make up the secondary endpoints.
The PELICAN trial with ultimately include a total of 10 investigational sites in the U.S. and 10 in Europe.
Source: ZKR Orthopedics

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







