
 Copy to clipboard
Copy to clipboard 
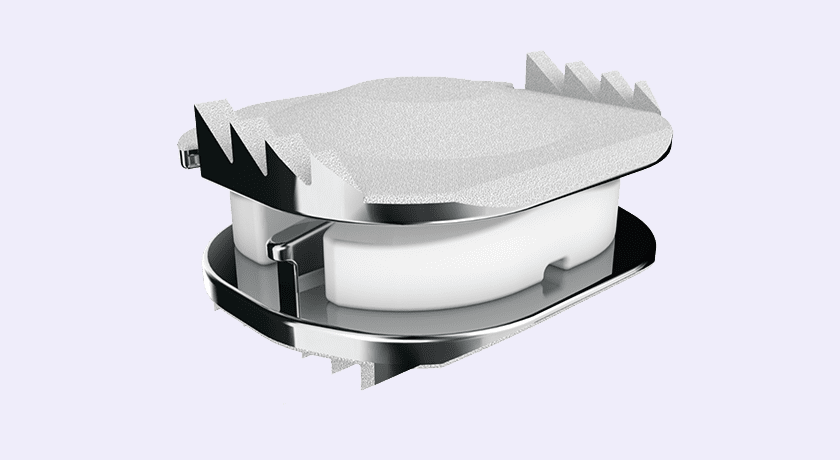
Over 200,000 of ZimVie’s Mobi-C Cervical Discs have been implanted worldwide. This includes patients treated in over 25 countries since the first surgery was completed with the device in France in 2004. In 2013, Mobi-C became the first cervical disc to win approval from FDA for the treatment of more than one level of the cervical spine.
FDA has determined Mobi-C to be statistically superior to fusion at seven years for two-level cervical disc replacement, based on the primary study endpoint of a prospective, concurrently controlled and randomized, multi-center clinical trial. At 10 years, all patient-reported outcomes were equivalent to or improved from seven years.
“Mobi-C is differentiated by substantial, long-term clinical data and we are proud to announce this milestone, reflecting tens of thousands of patient lives restored with our novel device,” said Rebecca Whitney, Global President of ZimVie Spine. “As we mark the ten-year anniversary of Mobi-C FDA approval for treatment of one and two levels of the cervical spine, we are excited to embrace 2023 as ‘the Year of Mobi-C’ by acknowledging and celebrating throughout the year the clinical success our surgeon customers have realized for their patients.”
Source: ZimVie
Over 200,000 of ZimVie's Mobi-C Cervical Discs have been implanted worldwide. This includes patients treated in over 25 countries since the first surgery was completed with the device in France in 2004. In 2013, Mobi-C became the first cervical disc to win approval from FDA for the treatment of more than one level of the cervical spine.
FDA...
Over 200,000 of ZimVie’s Mobi-C Cervical Discs have been implanted worldwide. This includes patients treated in over 25 countries since the first surgery was completed with the device in France in 2004. In 2013, Mobi-C became the first cervical disc to win approval from FDA for the treatment of more than one level of the cervical spine.
FDA has determined Mobi-C to be statistically superior to fusion at seven years for two-level cervical disc replacement, based on the primary study endpoint of a prospective, concurrently controlled and randomized, multi-center clinical trial. At 10 years, all patient-reported outcomes were equivalent to or improved from seven years.
“Mobi-C is differentiated by substantial, long-term clinical data and we are proud to announce this milestone, reflecting tens of thousands of patient lives restored with our novel device,” said Rebecca Whitney, Global President of ZimVie Spine. “As we mark the ten-year anniversary of Mobi-C FDA approval for treatment of one and two levels of the cervical spine, we are excited to embrace 2023 as ‘the Year of Mobi-C’ by acknowledging and celebrating throughout the year the clinical success our surgeon customers have realized for their patients.”
Source: ZimVie

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







