
 Copy to clipboard
Copy to clipboard 
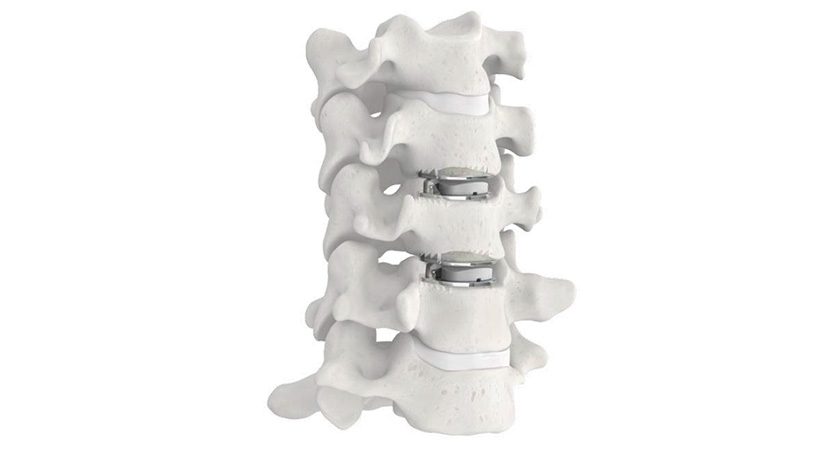
ZimVie was granted FDA approval of its Mobi-C Cervical Disc Hybrid Investigational Device Exemption (IDE) application. The decision authorizes ZimVie to begin enrolling U.S. patients in the study, which will follow patients who receive simultaneous cervical disc arthroplasty (CDA) and anterior cervical discectomy and fusion (ACDF) at adjacent levels between C3 and C7.
In some cases, the best two-level treatment may be just such a hybrid construct, where the disc replacement and fusion can be completed in one surgery, providing a clinical benefit to the patient and surgeon as well as an economic benefit to stakeholders in the healthcare delivery system.
Surgeons have implanted over 200,000 Mobi-C implants for cervical disc replacement at one level or two contiguous levels since 2004. In 2013, Mobi-C became the first cervical disc approved for one and two levels by FDA.
ZimVie intends to begin enrollment over the next several months and conduct the IDE study with multiple surgeons at six sites over the next five years.
“The decision to move forward with the Mobi-C hybrid study demonstrates our ongoing leadership and significant investment in continuing to develop the cervical arthroplasty market,” said Rebecca Whitney, Global President of ZimVie Spine. “We are committed to and passionate about driving the expansion of this market to provide a greater number of patients with the gift of motion. We are pleased to be at the forefront of important clinical studies to make motion preservation a reality for more patients.”
Source: ZimVie
ZimVie was granted FDA approval of its Mobi-C Cervical Disc Hybrid Investigational Device Exemption (IDE) application. The decision authorizes ZimVie to begin enrolling U.S. patients in the study, which will follow patients who receive simultaneous cervical disc arthroplasty (CDA) and anterior cervical discectomy and fusion (ACDF) at adjacent...
ZimVie was granted FDA approval of its Mobi-C Cervical Disc Hybrid Investigational Device Exemption (IDE) application. The decision authorizes ZimVie to begin enrolling U.S. patients in the study, which will follow patients who receive simultaneous cervical disc arthroplasty (CDA) and anterior cervical discectomy and fusion (ACDF) at adjacent levels between C3 and C7.
In some cases, the best two-level treatment may be just such a hybrid construct, where the disc replacement and fusion can be completed in one surgery, providing a clinical benefit to the patient and surgeon as well as an economic benefit to stakeholders in the healthcare delivery system.
Surgeons have implanted over 200,000 Mobi-C implants for cervical disc replacement at one level or two contiguous levels since 2004. In 2013, Mobi-C became the first cervical disc approved for one and two levels by FDA.
ZimVie intends to begin enrollment over the next several months and conduct the IDE study with multiple surgeons at six sites over the next five years.
“The decision to move forward with the Mobi-C hybrid study demonstrates our ongoing leadership and significant investment in continuing to develop the cervical arthroplasty market,” said Rebecca Whitney, Global President of ZimVie Spine. “We are committed to and passionate about driving the expansion of this market to provide a greater number of patients with the gift of motion. We are pleased to be at the forefront of important clinical studies to make motion preservation a reality for more patients.”
Source: ZimVie

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







