
 Copy to clipboard
Copy to clipboard 
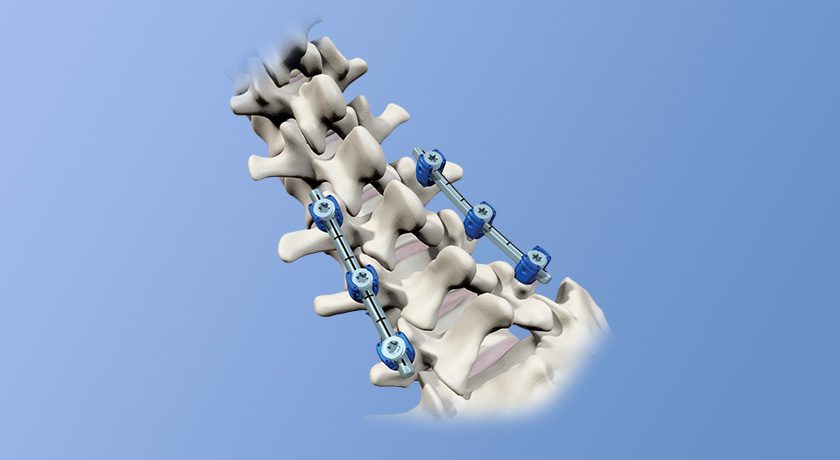
ZimVie received FDA 510(k) clearance for Vital Spinal Fixation, including instruments for use with Brainlab Spine & Trauma Navigation.
This follows the execution of the companies’ Development Cooperation Agreement to achieve compatibility between ZimVie’s Vital and Virage systems and Brainlab Spine & Trauma Navigation, which helps surgeons plan and execute spinal procedures, accurately place pedicle screws and minimize radiation exposure.
ZimVie plans to co-market Brainlab Spine & Trauma Navigation alongside its Vital and Virage lines, with first Vital sets expected to be launched in the United States in early 2024. Having secured that clearance, the company plans to submit a 510(k) application to FDA for Virage next year, as well.
Vital Spinal Fixation includes an optimized instrument and implant kit configuration for complex and degenerative thoracolumbar procedures designed to provide surgeons with the flexibility to utilize instrumentation based on their personal technique, preference and specific patient needs. A series of instruments and reference arrays designed to be compatible with Brainlab spine and trauma navigation technologies allows navigation during bone preparation and placement of pedicle screws during spinal surgery to assist the surgeon in precisely locating anatomical structures in either open or minimally invasive procedures.
Source: ZimVie
ZimVie received FDA 510(k) clearance for Vital Spinal Fixation, including instruments for use with Brainlab Spine & Trauma Navigation.
This follows the execution of the companies’ Development Cooperation Agreement to achieve compatibility between ZimVie’s Vital and Virage systems and Brainlab Spine & Trauma Navigation, which helps...
ZimVie received FDA 510(k) clearance for Vital Spinal Fixation, including instruments for use with Brainlab Spine & Trauma Navigation.
This follows the execution of the companies’ Development Cooperation Agreement to achieve compatibility between ZimVie’s Vital and Virage systems and Brainlab Spine & Trauma Navigation, which helps surgeons plan and execute spinal procedures, accurately place pedicle screws and minimize radiation exposure.
ZimVie plans to co-market Brainlab Spine & Trauma Navigation alongside its Vital and Virage lines, with first Vital sets expected to be launched in the United States in early 2024. Having secured that clearance, the company plans to submit a 510(k) application to FDA for Virage next year, as well.
Vital Spinal Fixation includes an optimized instrument and implant kit configuration for complex and degenerative thoracolumbar procedures designed to provide surgeons with the flexibility to utilize instrumentation based on their personal technique, preference and specific patient needs. A series of instruments and reference arrays designed to be compatible with Brainlab spine and trauma navigation technologies allows navigation during bone preparation and placement of pedicle screws during spinal surgery to assist the surgeon in precisely locating anatomical structures in either open or minimally invasive procedures.
Source: ZimVie

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







