Copy to clipboard
Copy to clipboard 
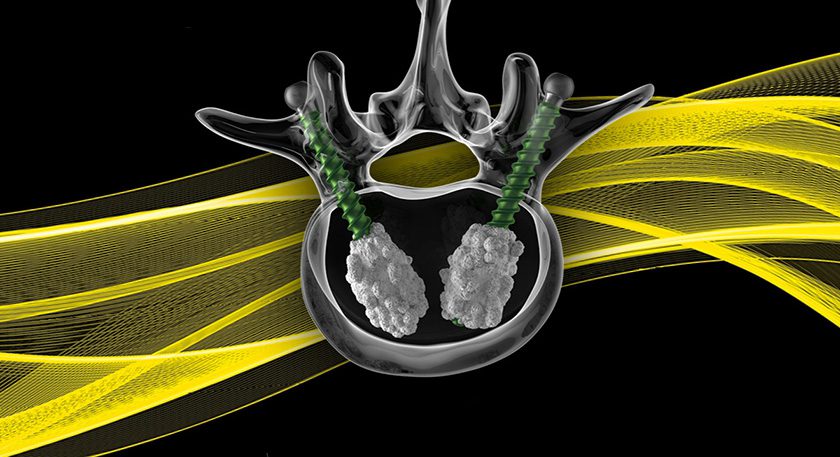
Zimmer Biomet is recalling SpF® PLUS-Mini and SpF XL IIb Implantable Spinal Fusion Stimulators due to potential cytotoxicity issues that were found during routine monitoring. This Class I recall affects units manufactured between 10/11/16 to 1/18/17, distributed from 3/28/17 to 4/6/17. In the U.S., 33 devices are recalled.
The implantable devices are designed for use during spinal fusion to increase the possibility of permanently connecting two or more bones of the spine.
Source: FDA.gov
Zimmer Biomet is recalling SpF® PLUS-Mini and SpF XL IIb Implantable Spinal Fusion Stimulators due to potential cytotoxicity issues that were found during routine monitoring. This Class I recall affects units manufactured between 10/11/16 to 1/18/17, distributed from 3/28/17 to 4/6/17. In the U.S., 33 devices are recalled.
The implantable...
Zimmer Biomet is recalling SpF® PLUS-Mini and SpF XL IIb Implantable Spinal Fusion Stimulators due to potential cytotoxicity issues that were found during routine monitoring. This Class I recall affects units manufactured between 10/11/16 to 1/18/17, distributed from 3/28/17 to 4/6/17. In the U.S., 33 devices are recalled.
The implantable devices are designed for use during spinal fusion to increase the possibility of permanently connecting two or more bones of the spine.
Source: FDA.gov

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.