Copy to clipboard
Copy to clipboard 
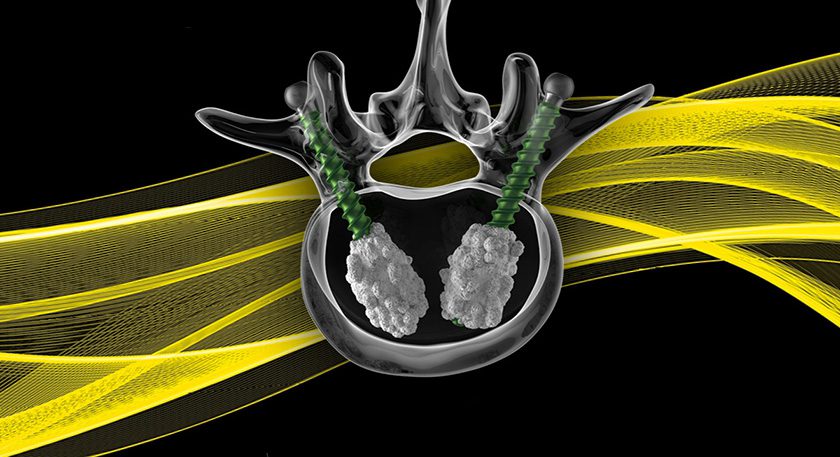
Zimmer Biomet was granted FDA 510(k) clearance to market the Persona® OsseoTi® Keel Tibia for cementless knee replacement. Persona OsseoTi is the latest addition to the Persona Knee System, and features a new porous version of the Persona anatomic tibia with Zimmer Biomet’s OsseoTi Porous Metal Technology, which uses anatomical data with 3D printing technology to build a structure that mimics the architecture of human cancellous bone. This material is combined with a keeled design to deliver stable initial and biological fixation.
Key features of Persona OsseoTi include an anatomic tibia for less micromotion and optimal bone coverage, and 3D-printed porous OsseoTi technology for biological fixation. The Persona OsseoTi Keel Tibia is also complemented with a new cemented implant option to enable versatility for the surgeon during the procedure.
“With an increasing number of surgeons opting for cementless procedures for their patients, we are excited to expand our market-leading Persona Knee portfolio with the Persona OsseoTi Keel Tibia…for performing a cementless total knee replacement,” said Ivan Tornos, Chief Operating Officer at Zimmer Biomet. “Adding the Persona OsseoTi Keel Tibia to our Persona Knee System allows surgeons to better address the needs of their patients with a comprehensive single system solution for a cementless or cemented application.”
Source: Zimmer Biomet
Zimmer Biomet was granted FDA 510(k) clearance to market the Persona® OsseoTi® Keel Tibia for cementless knee replacement. Persona OsseoTi is the latest addition to the Persona Knee System, and features a new porous version of the Persona anatomic tibia with Zimmer Biomet's OsseoTi Porous Metal Technology, which uses anatomical data with 3D...
Zimmer Biomet was granted FDA 510(k) clearance to market the Persona® OsseoTi® Keel Tibia for cementless knee replacement. Persona OsseoTi is the latest addition to the Persona Knee System, and features a new porous version of the Persona anatomic tibia with Zimmer Biomet’s OsseoTi Porous Metal Technology, which uses anatomical data with 3D printing technology to build a structure that mimics the architecture of human cancellous bone. This material is combined with a keeled design to deliver stable initial and biological fixation.
Key features of Persona OsseoTi include an anatomic tibia for less micromotion and optimal bone coverage, and 3D-printed porous OsseoTi technology for biological fixation. The Persona OsseoTi Keel Tibia is also complemented with a new cemented implant option to enable versatility for the surgeon during the procedure.
“With an increasing number of surgeons opting for cementless procedures for their patients, we are excited to expand our market-leading Persona Knee portfolio with the Persona OsseoTi Keel Tibia…for performing a cementless total knee replacement,” said Ivan Tornos, Chief Operating Officer at Zimmer Biomet. “Adding the Persona OsseoTi Keel Tibia to our Persona Knee System allows surgeons to better address the needs of their patients with a comprehensive single system solution for a cementless or cemented application.”
Source: Zimmer Biomet

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.