Copy to clipboard
Copy to clipboard 
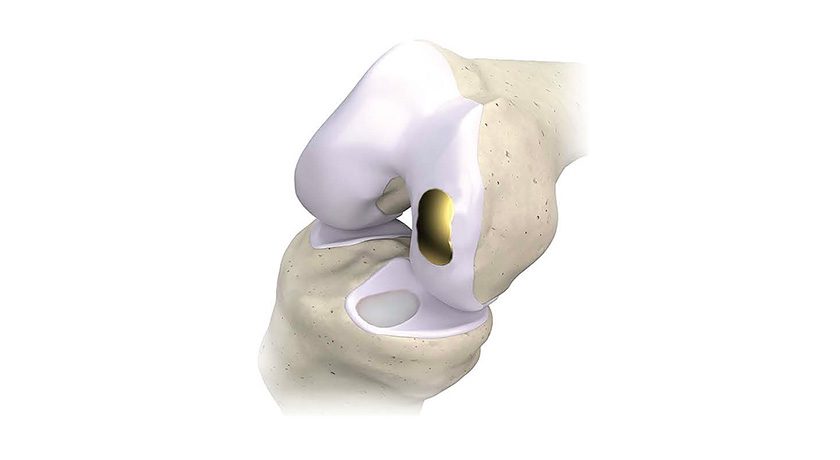
Zimmer Biomet received FDA 510(k) clearance to market the Zyston® Strut Open Titanium Interbody Spacer, the company’s first titanium spinal implant manufactured via 3D printing.
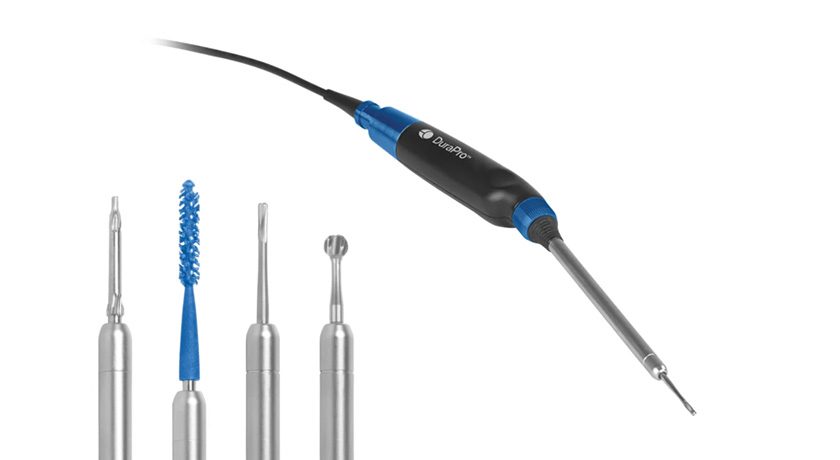
Zyston comprises a family of lumbar cages designed to enhance strength, graft capacity and visualization of the spacer, sized for a range of anatomies and surgical approaches. The system includes instruments for insertion, manipulation and implant removal.
Source: Zimmer Biomet
Zimmer Biomet received FDA 510(k) clearance to market the Zyston® Strut Open Titanium Interbody Spacer, the company's first titanium spinal implant manufactured via 3D printing.
Zyston comprises a family of lumbar cages designed to enhance strength, graft capacity and visualization of the spacer, sized for a range of anatomies...
Zimmer Biomet received FDA 510(k) clearance to market the Zyston® Strut Open Titanium Interbody Spacer, the company’s first titanium spinal implant manufactured via 3D printing.
Zyston comprises a family of lumbar cages designed to enhance strength, graft capacity and visualization of the spacer, sized for a range of anatomies and surgical approaches. The system includes instruments for insertion, manipulation and implant removal.
Source: Zimmer Biomet

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.