
 Copy to clipboard
Copy to clipboard 
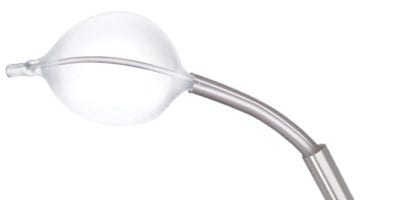
Zavation Medical Products doubled its kyphoplasty sales since its late-2018 acquisition of PanMed, and performed over 10,000 kyphoplasty procedures in 2019. Panmed’s minimally invasive products include a curved platform comprising InterV Flex and CurvePlus as well as traditional kyphoplasty systems.
The company will debut a new ergonomic instrument set within 1Q20.
Brad Risher, President of Interventional Spine, said, “The CurvePlus is the only FDA-approved kyphoplasty system that allows for both the balloon and cement to be passed through the same curved NiTi needle. We’re committed to offering innovative and differentiated products in conjunction with market leading customer service to best serve our surgeons and distribution network.”
Zavation has commercialized over 15 spine/biologic product families since 2012, and plans to launch 10 products over the course of 2020.
Zavation Medical Products doubled its kyphoplasty sales since its late-2018 acquisition of PanMed, and performed over 10,000 kyphoplasty procedures in 2019. Panmed's minimally invasive products include a curved platform comprising InterV Flex and CurvePlus as well as traditional kyphoplasty systems.
The company will debut a new ergonomic...
Zavation Medical Products doubled its kyphoplasty sales since its late-2018 acquisition of PanMed, and performed over 10,000 kyphoplasty procedures in 2019. Panmed’s minimally invasive products include a curved platform comprising InterV Flex and CurvePlus as well as traditional kyphoplasty systems.
The company will debut a new ergonomic instrument set within 1Q20.
Brad Risher, President of Interventional Spine, said, “The CurvePlus is the only FDA-approved kyphoplasty system that allows for both the balloon and cement to be passed through the same curved NiTi needle. We’re committed to offering innovative and differentiated products in conjunction with market leading customer service to best serve our surgeons and distribution network.”
Zavation has commercialized over 15 spine/biologic product families since 2012, and plans to launch 10 products over the course of 2020.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







