Copy to clipboard
Copy to clipboard 
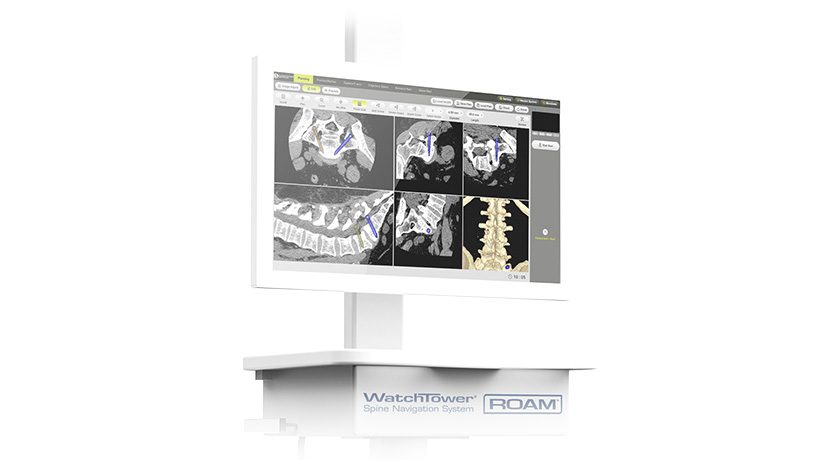
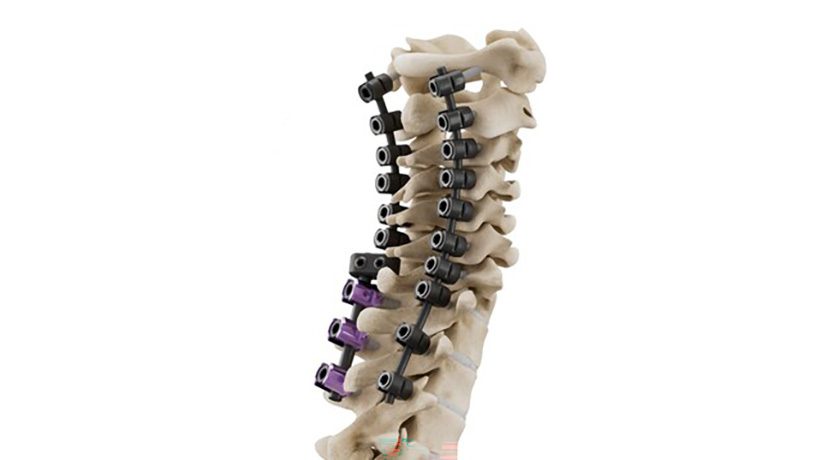
Xtant Medical received FDA 510(k) clearance for a posterior cervical screw indication on the Certex® Spinal Fixation System. Certex can be used with the Fortex® Pedicle and Xpress™ Minimally Invasive Pedicle Screw systems with rod-to-rod connectors and transition rods.
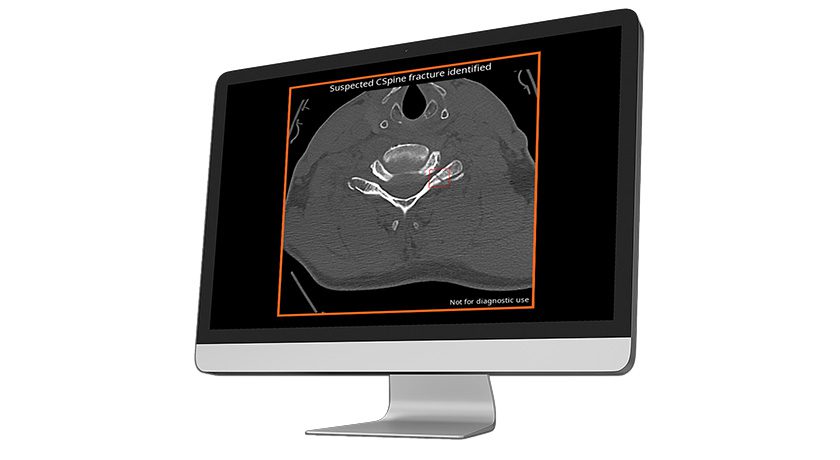
Certex is also intended to support the integrity of the spinal column without fusion for a limited time period in patients with advanced stage tumors of cervical spine, in whom life expectancy is medically determined to be of insufficient duration to permit achievement of fusion.
Certex received initial 510(k) clearance in 2012, with an indication for OCT use in 2013, which received CE Mark and global launch in 2015.
Sources: Xtant Medical Holdings, Inc., ORTHOWORLD
Xtant Medical received FDA 510(k) clearance for a posterior cervical screw indication on the Certex® Spinal Fixation System. Certex can be used with the Fortex® Pedicle and Xpress™ Minimally Invasive Pedicle Screw systems with rod-to-rod connectors and transition rods.
Certex is also intended to support the integrity of the spinal column...
Xtant Medical received FDA 510(k) clearance for a posterior cervical screw indication on the Certex® Spinal Fixation System. Certex can be used with the Fortex® Pedicle and Xpress™ Minimally Invasive Pedicle Screw systems with rod-to-rod connectors and transition rods.
Certex is also intended to support the integrity of the spinal column without fusion for a limited time period in patients with advanced stage tumors of cervical spine, in whom life expectancy is medically determined to be of insufficient duration to permit achievement of fusion.
Certex received initial 510(k) clearance in 2012, with an indication for OCT use in 2013, which received CE Mark and global launch in 2015.
Sources: Xtant Medical Holdings, Inc., ORTHOWORLD

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.