
 Copy to clipboard
Copy to clipboard 
Xtant Medical and Surgalign entered into a Definitive Agreement and subsequently closed on a transaction whereby Xtant acquired the Coflex and Cofix product lines from Surgalign for a total consideration of $17 million.
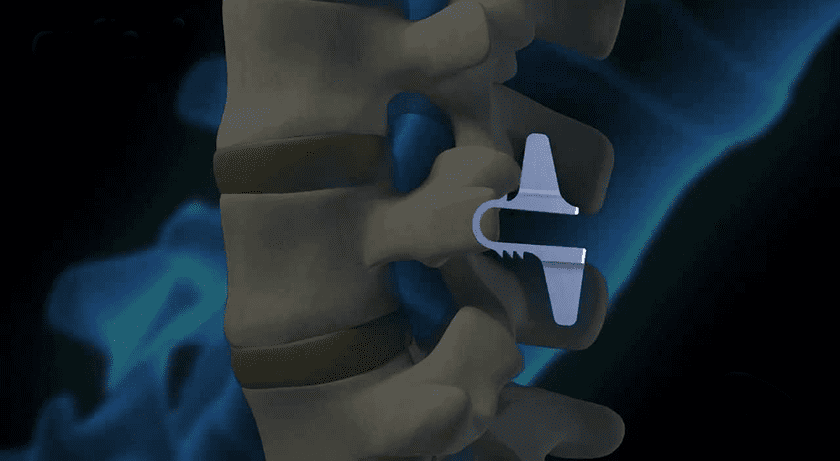
Coflex is an interlaminar stabilization device used after an open decompression that can be performed in various settings, offering a non-fusion treatment option for lumbar spinal stenosis (LSS) patients. Coflex is reportedly the only FDA premarket-approved implant for the treatment of LSS, and has established Ambulatory Surgery Center reimbursement. Cofix is a supplemental fixation device, which is a minimally invasive system intended for use on all levels of the lumbar spine.
Sean Browne, President and CEO of Xtant Medical, said, “Coupled with our less invasive Axle interspinous device and Silex SI Fusion product lines, Coflex augments our offering in the fast-growing segments of ASC and outpatient procedures. Aligning with our key growth pillars, this acquisition expands our footprint by adding new distributors and a significant number of trained surgeons to the company’s network. We expect these products to add approximately $14 million in annual revenue and attractive margins to enable Xtant to achieve profitability in the near future.”
Terry Rich, President and Chief Executive Officer of Surgalign, said, “Moving forward, we are focused on commercializing our HOLO Portal Surgical Guidance System, our soon to be launched HOLO AI Insights for research-use, and further developing our HOLO AI platform, while driving innovation in our remaining product lines to improve patient outcomes.”
Source: Surgalign and Xtant Medical
Xtant Medical and Surgalign entered into a Definitive Agreement and subsequently closed on a transaction whereby Xtant acquired the Coflex and Cofix product lines from Surgalign for a total consideration of $17 million.
Coflex is an interlaminar stabilization device used after an open decompression that can be performed in various settings,...
Xtant Medical and Surgalign entered into a Definitive Agreement and subsequently closed on a transaction whereby Xtant acquired the Coflex and Cofix product lines from Surgalign for a total consideration of $17 million.
Coflex is an interlaminar stabilization device used after an open decompression that can be performed in various settings, offering a non-fusion treatment option for lumbar spinal stenosis (LSS) patients. Coflex is reportedly the only FDA premarket-approved implant for the treatment of LSS, and has established Ambulatory Surgery Center reimbursement. Cofix is a supplemental fixation device, which is a minimally invasive system intended for use on all levels of the lumbar spine.
Sean Browne, President and CEO of Xtant Medical, said, “Coupled with our less invasive Axle interspinous device and Silex SI Fusion product lines, Coflex augments our offering in the fast-growing segments of ASC and outpatient procedures. Aligning with our key growth pillars, this acquisition expands our footprint by adding new distributors and a significant number of trained surgeons to the company’s network. We expect these products to add approximately $14 million in annual revenue and attractive margins to enable Xtant to achieve profitability in the near future.”
Terry Rich, President and Chief Executive Officer of Surgalign, said, “Moving forward, we are focused on commercializing our HOLO Portal Surgical Guidance System, our soon to be launched HOLO AI Insights for research-use, and further developing our HOLO AI platform, while driving innovation in our remaining product lines to improve patient outcomes.”
Source: Surgalign and Xtant Medical

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







