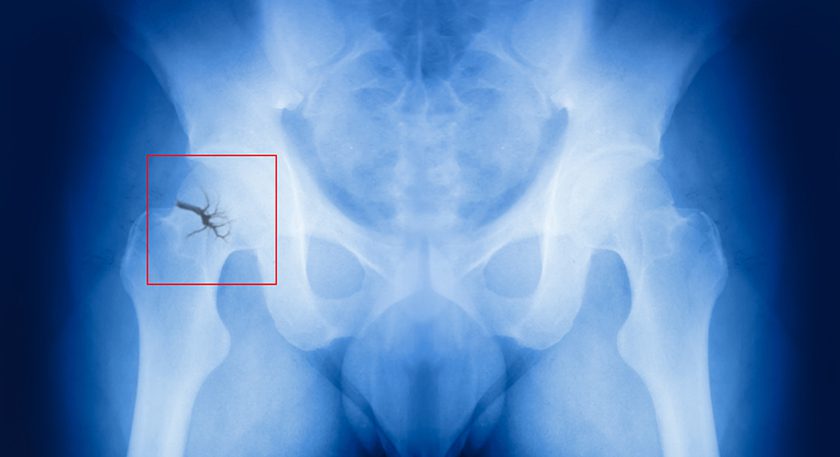






When surgeons perform orthopedic surgery with plates and screws, they often treat patients with difficult, compromised fixation scenarios that lead to complications and secondary surgeries when fixation between hardware and bone doesn’t hold. This risk is especially high in patients with chronic illnesses, those who take medications that impact bone (e.g., steroids or PPIs), or subjects with poor quality bone or poor healing.
To help avoid complications, surgeons plan meticulously and use a variety of techniques to achieve better fixation. For example, surgeons prescribe medications before surgery, use additional plates or larger screws, or even inject cement to strengthen the grip between screws and bone. However, many of the techniques available are not always appropriate, may be difficult to execute, and are accompanied by their own set of risks.
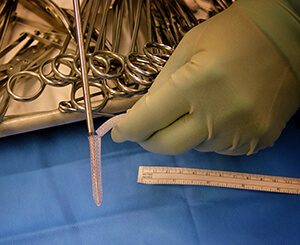

Ogmend Implant Enhancement
Woven Orthopedic Technologies has developed a small, innovative solution known as the Ogmend Implant Enhancement System to solve these issues. Ogmend acts like a wall anchor for human bone to help surgeons enhance fixation and achieve stability in challenging and unpredictable circumstances when screws have lost fixation. It’s been available to patients and surgeons in Europe for the past year and a half and is now making its way to the U.S. with recent approval from FDA for use in trauma surgery.
“It’s an incredibly simple technology and we’re excited to be releasing it for use in the U.S.,” said Brandon Bendes, Co-Founder and President of Woven Orthopedic Technologies. “It takes a surgeon 60 seconds to use and they’re able to do so along with the plate and screw systems they prefer. Most of us don’t appreciate the enormous amount of time surgeons spend planning how to achieve adequate fixation prior to surgery and then improvising to achieve it during surgery. It’s a pleasure to create something that helps simplify the process and make things faster, better, and easier.”
The Changing Challenges of Bone Fixation
When a surgeon enters a procedure, they need a number of tools to perform well. One of the challenges surgeons face today is the increasing rate of compromised fixation scenarios.
“As a society, we’re experiencing a large increase in the number of elderly patients who are living longer and remaining active during their lifetime,” Mr. Bendes said. “As a result, the procedures typically performed on younger, healthier patients are now being performed more frequently on older patients. For example, we’re hearing more and more stories about people in their 70s and 80s who are still active and receiving surgeries previously performed on patients in their 50s and 60s. Unfortunately, these patients just don’t have anatomy that is conducive to accepting hardware like younger patients would, and they don’t heal the same way anymore. As a result, they demand good outcomes but pose significant challenges to the surgeons who ultimately treat them.”
All of this is forcing surgeons to evolve how they perform surgery. Ogmend provides a tool to help in those worst-case, compromised fixation scenarios.
Keeping it Simple
The main purpose of plates and screws in orthopedic surgery is to maintain alignment and stability so that bone can heal. Without stability, a bone doesn’t heal. Fixation between screws and bone is essential to stability.
The Ogmend technology helps enhance stability at the screw-to-bone interface by buffering the forces between bone and screw, distributing load and enabling bony ingrowth to maintain fixation. It uses a well-known material and applies a proven solution in other industries (i.e., wall anchors in construction) to orthopedics.
“The wall anchor is a cost effective, low-risk product that’s been used for a long time, just not in orthopedics until now,” Mr. Bendes said.
The company doesn’t sell screws and isn’t trying to replace the screw systems available on the market today. Rather, the goal is to help existing systems function even better in extremely difficult circumstances.
Mr. Bendes gives the example of a surgeon performing proximal femoral fracture repair.


Ogmend – A tool to enhance fixation
“Depending on the patient, screw cut-out in proximal femoral fracture repair surgery can occur frequently,” he said. “Take an osteoporotic patient, for example. Oftentimes, a surgeon may try to get an additional point of contact in the femoral neck. Perhaps they’ll inject cement. Or in more difficult fixation scenarios, they may just progress straight to arthroplasty (hip replacement) because there are no better options. These are scenarios surgeons see all the time and while the goal is to perform the least disruptive surgery, they also have to balance that with achieving the best results. So, what we’re trying to do at Woven is provide surgeons with an additional tool to enhance fixation so that they can still use the more conservative approaches.”
Current Market Status
The Ogmend Implant Enhancement System received CE Mark approval for spine surgery and has been available in Europe since 2019. Initial market introduction was met with significant success, and this month the company received the coveted “Spine Technology of the Year” award from Orthopedics This Week (OTW).
“It was a great surprise and honor to receive the award from OTW,” Mr. Bendes said. “The judges are independent clinicians who represent unbiased, professional opinions and who are ultimately the end users of these products. The fact they’ve placed their faith in Ogmend is a big vote of confidence for the product’s importance.”
Recently, Ogmend also received FDA de novo clearance for use in the U.S. in long bone trauma surgery. The company plans to use the same three-phased approach to enter the U.S. market as it is doing in Europe.
“Phase one is an introduction, where we work closely with surgeons to collect data,” Mr. Bendes said. “We want to make sure that we learn where it works best and what we can do to maximize successful outcomes for surgeons and patients. That means identifying the ideal patients, understanding where and when it’s used most, and communicating pearls and pitfalls to surgeon users.”
The company commenced Phase I launch in Europe in 2019 by entering the U.K., Spain and Italy in a select number of hospitals. Sales and use grew by more than 500% during the year and have already eclipsed the 500 unit mark. In all units implanted to date, Ogmend has achieved 100% success with no device related complications or revisions.
“We tested the device rigorously in worst-case scenarios so that we could be confident it would work well,” Mr. Bendes said. “But I will admit that if you told me we’d have 100% success after more than a year on the market, I would’ve told you that was impossible. Perfection is unsustainable but it’s a great testament to the effectiveness of the product thus far.”
The company hopes to achieve similar results in the U.S. as they enter Phase I introduction. Over the next few years, the company will then execute Phase II, which focuses on increasing adoption, and Phase III, which focuses on selling at scale.
They also plan to enter additional markets and expand indications for use. “The concept behind the technology could apply anywhere a screw is used,” Mr. Bendes said. “It could eventually apply to dental, small bone, reconstruction, sports medicine, craniomaxillofacial, you name it.”
Once completed, Mr. Bendes sees the company facing two likely future scenarios.
“There are two ways this can go. One is, we start expanding across multiple indications and become the wall anchor for the orthopedic world. The other is, we partner with companies to offer Ogmend exclusively in conjunction with specific systems. Ultimately, the goal is to provide surgeons with the best solutions for compromised fixation scenarios. The fixation systems available on the market today are fantastic. Together, we can continue creating the right tools to make patient care even better.”
When surgeons perform orthopedic surgery with plates and screws, they often treat patients with difficult, compromised fixation scenarios that lead to complications and secondary surgeries when fixation between hardware and bone doesn’t hold. This risk is especially high in patients with chronic illnesses, those who take medications that impact...
When surgeons perform orthopedic surgery with plates and screws, they often treat patients with difficult, compromised fixation scenarios that lead to complications and secondary surgeries when fixation between hardware and bone doesn’t hold. This risk is especially high in patients with chronic illnesses, those who take medications that impact bone (e.g., steroids or PPIs), or subjects with poor quality bone or poor healing.
To help avoid complications, surgeons plan meticulously and use a variety of techniques to achieve better fixation. For example, surgeons prescribe medications before surgery, use additional plates or larger screws, or even inject cement to strengthen the grip between screws and bone. However, many of the techniques available are not always appropriate, may be difficult to execute, and are accompanied by their own set of risks.


Ogmend Implant Enhancement
Woven Orthopedic Technologies has developed a small, innovative solution known as the Ogmend Implant Enhancement System to solve these issues. Ogmend acts like a wall anchor for human bone to help surgeons enhance fixation and achieve stability in challenging and unpredictable circumstances when screws have lost fixation. It’s been available to patients and surgeons in Europe for the past year and a half and is now making its way to the U.S. with recent approval from FDA for use in trauma surgery.
“It’s an incredibly simple technology and we’re excited to be releasing it for use in the U.S.,” said Brandon Bendes, Co-Founder and President of Woven Orthopedic Technologies. “It takes a surgeon 60 seconds to use and they’re able to do so along with the plate and screw systems they prefer. Most of us don’t appreciate the enormous amount of time surgeons spend planning how to achieve adequate fixation prior to surgery and then improvising to achieve it during surgery. It’s a pleasure to create something that helps simplify the process and make things faster, better, and easier.”
The Changing Challenges of Bone Fixation
When a surgeon enters a procedure, they need a number of tools to perform well. One of the challenges surgeons face today is the increasing rate of compromised fixation scenarios.
“As a society, we’re experiencing a large increase in the number of elderly patients who are living longer and remaining active during their lifetime,” Mr. Bendes said. “As a result, the procedures typically performed on younger, healthier patients are now being performed more frequently on older patients. For example, we’re hearing more and more stories about people in their 70s and 80s who are still active and receiving surgeries previously performed on patients in their 50s and 60s. Unfortunately, these patients just don’t have anatomy that is conducive to accepting hardware like younger patients would, and they don’t heal the same way anymore. As a result, they demand good outcomes but pose significant challenges to the surgeons who ultimately treat them.”
All of this is forcing surgeons to evolve how they perform surgery. Ogmend provides a tool to help in those worst-case, compromised fixation scenarios.
Keeping it Simple
The main purpose of plates and screws in orthopedic surgery is to maintain alignment and stability so that bone can heal. Without stability, a bone doesn’t heal. Fixation between screws and bone is essential to stability.
The Ogmend technology helps enhance stability at the screw-to-bone interface by buffering the forces between bone and screw, distributing load and enabling bony ingrowth to maintain fixation. It uses a well-known material and applies a proven solution in other industries (i.e., wall anchors in construction) to orthopedics.
“The wall anchor is a cost effective, low-risk product that’s been used for a long time, just not in orthopedics until now,” Mr. Bendes said.
The company doesn’t sell screws and isn’t trying to replace the screw systems available on the market today. Rather, the goal is to help existing systems function even better in extremely difficult circumstances.
Mr. Bendes gives the example of a surgeon performing proximal femoral fracture repair.


Ogmend – A tool to enhance fixation
“Depending on the patient, screw cut-out in proximal femoral fracture repair surgery can occur frequently,” he said. “Take an osteoporotic patient, for example. Oftentimes, a surgeon may try to get an additional point of contact in the femoral neck. Perhaps they’ll inject cement. Or in more difficult fixation scenarios, they may just progress straight to arthroplasty (hip replacement) because there are no better options. These are scenarios surgeons see all the time and while the goal is to perform the least disruptive surgery, they also have to balance that with achieving the best results. So, what we’re trying to do at Woven is provide surgeons with an additional tool to enhance fixation so that they can still use the more conservative approaches.”
Current Market Status
The Ogmend Implant Enhancement System received CE Mark approval for spine surgery and has been available in Europe since 2019. Initial market introduction was met with significant success, and this month the company received the coveted “Spine Technology of the Year” award from Orthopedics This Week (OTW).
“It was a great surprise and honor to receive the award from OTW,” Mr. Bendes said. “The judges are independent clinicians who represent unbiased, professional opinions and who are ultimately the end users of these products. The fact they’ve placed their faith in Ogmend is a big vote of confidence for the product’s importance.”
Recently, Ogmend also received FDA de novo clearance for use in the U.S. in long bone trauma surgery. The company plans to use the same three-phased approach to enter the U.S. market as it is doing in Europe.
“Phase one is an introduction, where we work closely with surgeons to collect data,” Mr. Bendes said. “We want to make sure that we learn where it works best and what we can do to maximize successful outcomes for surgeons and patients. That means identifying the ideal patients, understanding where and when it’s used most, and communicating pearls and pitfalls to surgeon users.”
The company commenced Phase I launch in Europe in 2019 by entering the U.K., Spain and Italy in a select number of hospitals. Sales and use grew by more than 500% during the year and have already eclipsed the 500 unit mark. In all units implanted to date, Ogmend has achieved 100% success with no device related complications or revisions.
“We tested the device rigorously in worst-case scenarios so that we could be confident it would work well,” Mr. Bendes said. “But I will admit that if you told me we’d have 100% success after more than a year on the market, I would’ve told you that was impossible. Perfection is unsustainable but it’s a great testament to the effectiveness of the product thus far.”
The company hopes to achieve similar results in the U.S. as they enter Phase I introduction. Over the next few years, the company will then execute Phase II, which focuses on increasing adoption, and Phase III, which focuses on selling at scale.
They also plan to enter additional markets and expand indications for use. “The concept behind the technology could apply anywhere a screw is used,” Mr. Bendes said. “It could eventually apply to dental, small bone, reconstruction, sports medicine, craniomaxillofacial, you name it.”
Once completed, Mr. Bendes sees the company facing two likely future scenarios.
“There are two ways this can go. One is, we start expanding across multiple indications and become the wall anchor for the orthopedic world. The other is, we partner with companies to offer Ogmend exclusively in conjunction with specific systems. Ultimately, the goal is to provide surgeons with the best solutions for compromised fixation scenarios. The fixation systems available on the market today are fantastic. Together, we can continue creating the right tools to make patient care even better.”


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
HT
Heather Tunstall is an ORTHOWORLD Contributor and owner of Tunstall Content.