
 Copy to clipboard
Copy to clipboard 
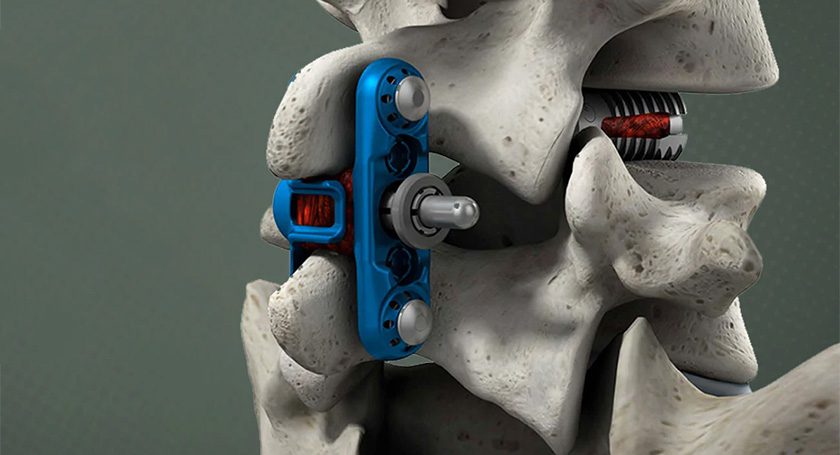
Wenzel Spine announced FDA 510(k) clearance for their primaLOK SP spinal fusion technology. New clinical indications allow for primaLOK SP to be used at multiple levels and to treat patients with lumbar spinal stenosis.
Key features and benefits of primaLOK SP include:
- A locking mechanism that ensures secure stabilization, reducing the risk of migration and providing stability during the critical fusion process.
- Flexibility to accommodate various patient anatomies and offer surgeons the ability to tailor each procedure to individual needs.
- A minimally invasive approach to minimize tissue disruption, potentially leading to reduced postoperative pain, shorter hospital stays and quicker patient recovery.
- Enhanced radiographic visibility, allowing surgeons to assess implant placement and fusion progress.
Source: Wenzel Spine
Wenzel Spine announced FDA 510(k) clearance for their primaLOK SP spinal fusion technology. New clinical indications allow for primaLOK SP to be used at multiple levels and to treat patients with lumbar spinal stenosis.
Key features and benefits of primaLOK SP include:
A locking mechanism that ensures secure stabilization, reducing the...
Wenzel Spine announced FDA 510(k) clearance for their primaLOK SP spinal fusion technology. New clinical indications allow for primaLOK SP to be used at multiple levels and to treat patients with lumbar spinal stenosis.
Key features and benefits of primaLOK SP include:
- A locking mechanism that ensures secure stabilization, reducing the risk of migration and providing stability during the critical fusion process.
- Flexibility to accommodate various patient anatomies and offer surgeons the ability to tailor each procedure to individual needs.
- A minimally invasive approach to minimize tissue disruption, potentially leading to reduced postoperative pain, shorter hospital stays and quicker patient recovery.
- Enhanced radiographic visibility, allowing surgeons to assess implant placement and fusion progress.
Source: Wenzel Spine

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







