
 Copy to clipboard
Copy to clipboard 
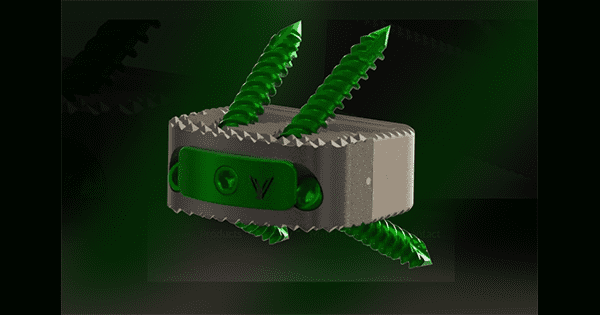
VySpine received FDA 510(k) clearance to market the LumiVy Lumbar IBF System, designed for use after lumbar discectomy in fusion procedures.
The LumiVy Lumbar IBF System features lumbar interbody fusion devices made from either PEEK Optima LT1 or PEEK Optima HA-Enhanced. PEEK Optima LT1 has been the standard for interbody devices for decade,s due to its radiolucency and a modulus of elasticity close to that of natural bone. PEEK Optima HA-Enhanced has embedded Hydroxyapatite fibers to create better bony apposition to the implant.
The LumiVy Lumbar IBF System is offered in numerous footprints and heights, and addresses a full range of lumbar interbody approaches including anterior, oblique anterior, lateral, oblique posterior, posterior and transforaminal. LumiVy implants are available in a wide range of lordosis. The LumiVy Lumbar IBF System also features IBF-S implants, which have self-drilling screws to aid in anchoring the device directly to the bone.
Source: VySpine
VySpine received FDA 510(k) clearance to market the LumiVy Lumbar IBF System, designed for use after lumbar discectomy in fusion procedures.
The LumiVy Lumbar IBF System features lumbar interbody fusion devices made from either PEEK Optima LT1 or PEEK Optima HA-Enhanced. PEEK Optima LT1 has been the standard for interbody devices for decade,s...
VySpine received FDA 510(k) clearance to market the LumiVy Lumbar IBF System, designed for use after lumbar discectomy in fusion procedures.
The LumiVy Lumbar IBF System features lumbar interbody fusion devices made from either PEEK Optima LT1 or PEEK Optima HA-Enhanced. PEEK Optima LT1 has been the standard for interbody devices for decade,s due to its radiolucency and a modulus of elasticity close to that of natural bone. PEEK Optima HA-Enhanced has embedded Hydroxyapatite fibers to create better bony apposition to the implant.
The LumiVy Lumbar IBF System is offered in numerous footprints and heights, and addresses a full range of lumbar interbody approaches including anterior, oblique anterior, lateral, oblique posterior, posterior and transforaminal. LumiVy implants are available in a wide range of lordosis. The LumiVy Lumbar IBF System also features IBF-S implants, which have self-drilling screws to aid in anchoring the device directly to the bone.
Source: VySpine

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







