
 Copy to clipboard
Copy to clipboard 
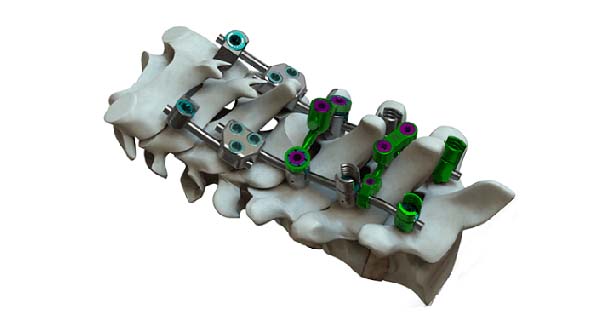
VySpine received FDA 510(k) clearance to market the VySpan Posterior Cervical Thoracic System. This is the company’s first FDA-cleared product.
VySpan PCT features various screw and hook options, multiple transition rods and revolutionary crosslink versatility, and offers a variety of implant options for the thoracic spine.
The VySpan PCT System features fixed and polyaxial head styles, each with a reduction option, that can be paired with either standard or smooth shank bone screws and a variety of hooks and rods.
VySpan PCT also offers a unique assortment of rod-to-rod and cross connectors, including a novel head-to-head double joint cross connector, as well as three- and four-point cross connectors for extraordinary versatility. Rod-to-rod connectors allow the VySpan PCT System to connect with larger Ø5.5mm and Ø6.0mm rods and includes transition rods, which taper from Ø3.5mm to either Ø5.5mm or Ø6.0mm.
“The VySpan PCT System is the first of many highly differentiated systems being developed by VySpine,” said Tom McLeer, CEO. “Using new materials and creative surgeon input, we are building exceptional quality, flexibility, and pricing into all our products. This is just the beginning of the exciting, innovative product launches scheduled for early 2022.”
Source: VySpine
VySpine received FDA 510(k) clearance to market the VySpan Posterior Cervical Thoracic System. This is the company's first FDA-cleared product.
VySpan PCT features various screw and hook options, multiple transition rods and revolutionary crosslink versatility, and offers a variety of implant options for the thoracic spine.
The VySpan PCT...
VySpine received FDA 510(k) clearance to market the VySpan Posterior Cervical Thoracic System. This is the company’s first FDA-cleared product.
VySpan PCT features various screw and hook options, multiple transition rods and revolutionary crosslink versatility, and offers a variety of implant options for the thoracic spine.
The VySpan PCT System features fixed and polyaxial head styles, each with a reduction option, that can be paired with either standard or smooth shank bone screws and a variety of hooks and rods.
VySpan PCT also offers a unique assortment of rod-to-rod and cross connectors, including a novel head-to-head double joint cross connector, as well as three- and four-point cross connectors for extraordinary versatility. Rod-to-rod connectors allow the VySpan PCT System to connect with larger Ø5.5mm and Ø6.0mm rods and includes transition rods, which taper from Ø3.5mm to either Ø5.5mm or Ø6.0mm.
“The VySpan PCT System is the first of many highly differentiated systems being developed by VySpine,” said Tom McLeer, CEO. “Using new materials and creative surgeon input, we are building exceptional quality, flexibility, and pricing into all our products. This is just the beginning of the exciting, innovative product launches scheduled for early 2022.”
Source: VySpine

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







