
 Copy to clipboard
Copy to clipboard 
VGI Medical commenced commercial launch of the VerteLP® Lateral Interbody Fusion system. VerteLP received FDA 510(k) clearance in 1Q16, and alpha launched one month following.
VerteLP is designed to preserve anatomy and avoid interference with previously-placed pedicle screws. Its bilateral plate fixation technique is intended to limit axial rotation and lateral bending.
Other lateral-approach systems in the news include NuVasive’s launch of Lateral ALIF, XLIF Crestline™ and Lateral MAS® Fixation and Spineology’s introduction of a prospective, postmarket lateral interbody fusion study to assess patient outcomes with the Duo™ Lumbar system.
Sources: VGI Medical, LLC; ORTHOWORLD Inc.
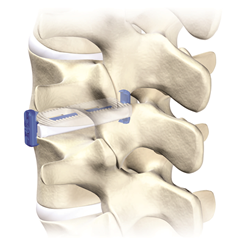
Image courtesy of VGI Medical, LLC
VGI Medical commenced commercial launch of the VerteLP® Lateral Interbody Fusion system. VerteLP received FDA 510(k) clearance in 1Q16, and alpha launched one month following.
VerteLP is designed to preserve anatomy and avoid interference with previously-placed pedicle screws. Its bilateral plate fixation technique is intended to limit axial...
VGI Medical commenced commercial launch of the VerteLP® Lateral Interbody Fusion system. VerteLP received FDA 510(k) clearance in 1Q16, and alpha launched one month following.
VerteLP is designed to preserve anatomy and avoid interference with previously-placed pedicle screws. Its bilateral plate fixation technique is intended to limit axial rotation and lateral bending.
Other lateral-approach systems in the news include NuVasive’s launch of Lateral ALIF, XLIF Crestline™ and Lateral MAS® Fixation and Spineology’s introduction of a prospective, postmarket lateral interbody fusion study to assess patient outcomes with the Duo™ Lumbar system.
Sources: VGI Medical, LLC; ORTHOWORLD Inc.

Image courtesy of VGI Medical, LLC

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







