
 Copy to clipboard
Copy to clipboard 
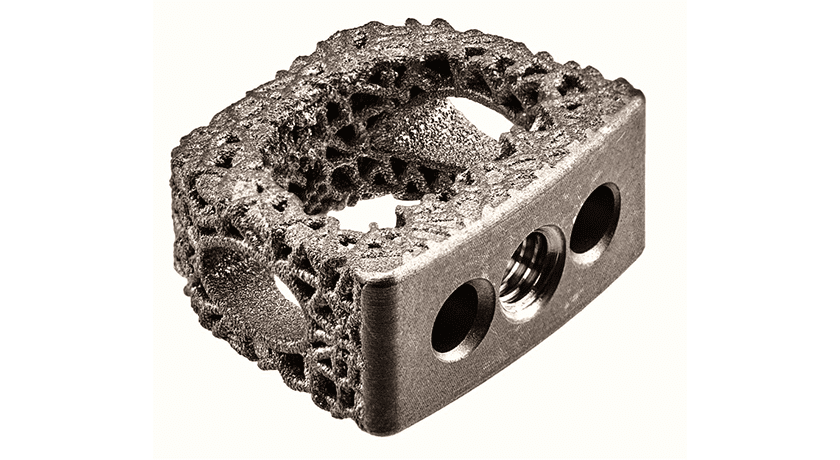
ulrich medical USA received FDA 510(k) clearance to market its Flux-C 3D printed porous titanium cervical interbody device.
Flux-C is manufactured using a 3D printing process called direct metal laser sintering. The interbodies are available in multiple parallel and lordotic options in various heights. These porous titanium devices are designed with a large graft window and a side window to allow for improved radiographic imaging.
“Surgeons have many options for cervical interbodies. The Flux-C porous titanium device offers one of the best in class with superior endplate contact and spaces for generous inter-device bone grafting. It is a welcomed complement to their superior array of expandable cages,” said Patrick Maloney, M.D. newest member of ulrich Medical USA’s Surgeon Advisory Board and its recently established Director of Deformity.
Eric Lucas, Ph.D., ulrich Medical USA’s Director of Technology, said, “We are continuing to develop procedural solutions for reconstruction of all spinal pathologies in collaboration with our Surgeon Advisory Board. We strive to help our surgeons and distributors achieve New Heights and Beyond with integrity, through excellence in design, manufacturing and craftsmanship.”
Source: ulrich Medical USA
ulrich medical USA received FDA 510(k) clearance to market its Flux-C 3D printed porous titanium cervical interbody device.
Flux-C is manufactured using a 3D printing process called direct metal laser sintering. The interbodies are available in multiple parallel and lordotic options in various heights. These porous titanium devices are...
ulrich medical USA received FDA 510(k) clearance to market its Flux-C 3D printed porous titanium cervical interbody device.
Flux-C is manufactured using a 3D printing process called direct metal laser sintering. The interbodies are available in multiple parallel and lordotic options in various heights. These porous titanium devices are designed with a large graft window and a side window to allow for improved radiographic imaging.
“Surgeons have many options for cervical interbodies. The Flux-C porous titanium device offers one of the best in class with superior endplate contact and spaces for generous inter-device bone grafting. It is a welcomed complement to their superior array of expandable cages,” said Patrick Maloney, M.D. newest member of ulrich Medical USA’s Surgeon Advisory Board and its recently established Director of Deformity.
Eric Lucas, Ph.D., ulrich Medical USA’s Director of Technology, said, “We are continuing to develop procedural solutions for reconstruction of all spinal pathologies in collaboration with our Surgeon Advisory Board. We strive to help our surgeons and distributors achieve New Heights and Beyond with integrity, through excellence in design, manufacturing and craftsmanship.”
Source: ulrich Medical USA

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







