
 Copy to clipboard
Copy to clipboard 
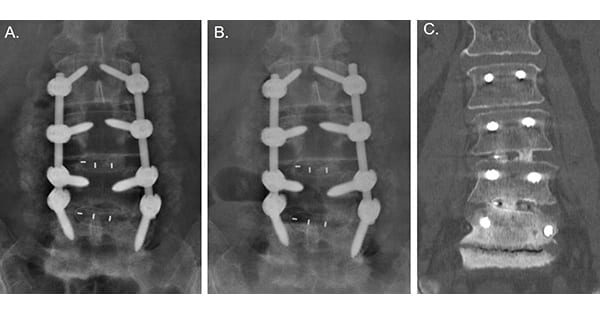
Tyber Medical launched a clinical study of its titanium-integrated interbody fusion devices for use in the cervical and lumbar spine for degenerative disc disease, stenosis, fractural repair and other degenerative and trauma-related conditions.
The devices in Tyber Medical’s clinical registry are PEEK devices that offer titanium integration for bone formation between the interbody device and surrounding tissues. Early osteo-integration of the devices and segment stability are being observed during patient recovery.
Tyber Medical fills gaps in existing distribution networks by providing design, engineering, quality management and regulatory support. “The significant investment we’re making in prospective and retrospective data collection and our leading MDR strategy provides customers with outcome data that supports the safety and effectiveness of our devices for domestic and international use,” said Tyber Medical Director of Clinical Research, Hallie Murray, PhD.
Tyber Medical launched a clinical study of its titanium-integrated interbody fusion devices for use in the cervical and lumbar spine for degenerative disc disease, stenosis, fractural repair and other degenerative and trauma-related conditions.
The devices in Tyber Medical's clinical registry are PEEK devices that offer titanium...
Tyber Medical launched a clinical study of its titanium-integrated interbody fusion devices for use in the cervical and lumbar spine for degenerative disc disease, stenosis, fractural repair and other degenerative and trauma-related conditions.
The devices in Tyber Medical’s clinical registry are PEEK devices that offer titanium integration for bone formation between the interbody device and surrounding tissues. Early osteo-integration of the devices and segment stability are being observed during patient recovery.
Tyber Medical fills gaps in existing distribution networks by providing design, engineering, quality management and regulatory support. “The significant investment we’re making in prospective and retrospective data collection and our leading MDR strategy provides customers with outcome data that supports the safety and effectiveness of our devices for domestic and international use,” said Tyber Medical Director of Clinical Research, Hallie Murray, PhD.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







