
 Copy to clipboard
Copy to clipboard 
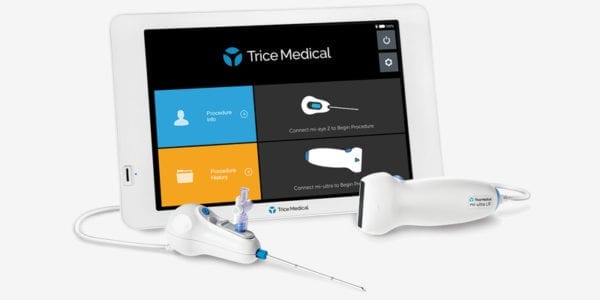
Trice Medical received FDA 510(k) clearance to market mi-eye 2, a disposable needle with integrated camera to support diagnosis of joint injury.
The next-generation iteration of mi-eye technology is designed for use in diagnostic and operative arthroscopic and endoscopic procedures to illuminate an interior cavity through a natural or surgical opening. mi-eye 2 also allows injection or aspiration under direct visualization.
Platform enhancements include improved resolution, field of view, depth of field and overall visualization.
Sources: Trice Medical; ORTHOWORLD Inc.
Trice Medical received FDA 510(k) clearance to market mi-eye 2, a disposable needle with integrated camera to support diagnosis of joint injury.
The next-generation iteration of mi-eye technology is designed for use in diagnostic and operative arthroscopic and endoscopic procedures to illuminate an interior cavity through a natural or surgical...
Trice Medical received FDA 510(k) clearance to market mi-eye 2, a disposable needle with integrated camera to support diagnosis of joint injury.
The next-generation iteration of mi-eye technology is designed for use in diagnostic and operative arthroscopic and endoscopic procedures to illuminate an interior cavity through a natural or surgical opening. mi-eye 2 also allows injection or aspiration under direct visualization.
Platform enhancements include improved resolution, field of view, depth of field and overall visualization.
Sources: Trice Medical; ORTHOWORLD Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







