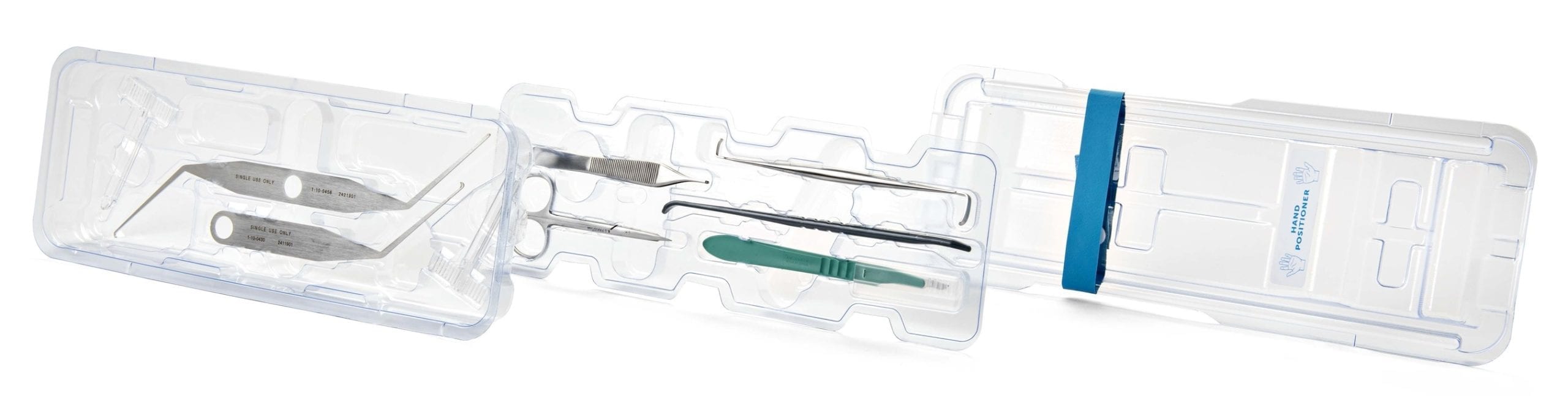






In the U.S., Trice Medical launched Seg-WAY ECTR-d, the first fully disposable endoscopic carpal tunnel release system.
The ECTR-d kit enables endoscopic carpal tunnel procedures to be performed in any site of care, with a limited burden on healthcare resources. Patients can be treated in the physician’s office under local anesthetic, reducing complexity, time and cost. As it is a sterile packaged, one-time use system, ECTR-d may reduce infection risk.
Seg-WAY ECTR-d features all instruments to complete an endoscopic carpal tunnel release procedure, and can be utilized with a disposable 2.3 mm camera such as the company’s mi-eye angled disposable camera. Newly designed guides can also accommodate a traditional 4.0mm arthroscope if preferred by the surgeon.
“Over 700,000 Americans have a carpal tunnel release procedure performed annually in the U.S. Due to the need to have sterilization capabilities and access to expensive arthroscopy towers, all of the endoscopic procedures to date have been done in the Operating Rooms of Hospitals and ASC’s. With the backlog of elective procedures created by COVID-19, those same OR’s are about to face a level of demand that we have seen before. And now, with just a tablet and two sterile boxes, a surgeon can complete the procedure, thereby limiting the burden on precious resources”, said Mark Foster, President and CEO of Trice Medical.
Trice acquired SegWAY Orthopaedics in 2019 as a complement to its arthroscopic camera technology. SegWAY’s portfolio also included products to treat cubital tunnel syndrome, gastrocnemius equinus contracture and plantar fasciitis.
In the U.S., Trice Medical launched Seg-WAY ECTR-d, the first fully disposable endoscopic carpal tunnel release system.
The ECTR-d kit enables endoscopic carpal tunnel procedures to be performed in any site of care, with a limited burden on healthcare resources. Patients can be treated in the physician's office under local anesthetic, reducing...
In the U.S., Trice Medical launched Seg-WAY ECTR-d, the first fully disposable endoscopic carpal tunnel release system.
The ECTR-d kit enables endoscopic carpal tunnel procedures to be performed in any site of care, with a limited burden on healthcare resources. Patients can be treated in the physician’s office under local anesthetic, reducing complexity, time and cost. As it is a sterile packaged, one-time use system, ECTR-d may reduce infection risk.
Seg-WAY ECTR-d features all instruments to complete an endoscopic carpal tunnel release procedure, and can be utilized with a disposable 2.3 mm camera such as the company’s mi-eye angled disposable camera. Newly designed guides can also accommodate a traditional 4.0mm arthroscope if preferred by the surgeon.
“Over 700,000 Americans have a carpal tunnel release procedure performed annually in the U.S. Due to the need to have sterilization capabilities and access to expensive arthroscopy towers, all of the endoscopic procedures to date have been done in the Operating Rooms of Hospitals and ASC’s. With the backlog of elective procedures created by COVID-19, those same OR’s are about to face a level of demand that we have seen before. And now, with just a tablet and two sterile boxes, a surgeon can complete the procedure, thereby limiting the burden on precious resources”, said Mark Foster, President and CEO of Trice Medical.
Trice acquired SegWAY Orthopaedics in 2019 as a complement to its arthroscopic camera technology. SegWAY’s portfolio also included products to treat cubital tunnel syndrome, gastrocnemius equinus contracture and plantar fasciitis.


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







