
 Copy to clipboard
Copy to clipboard 
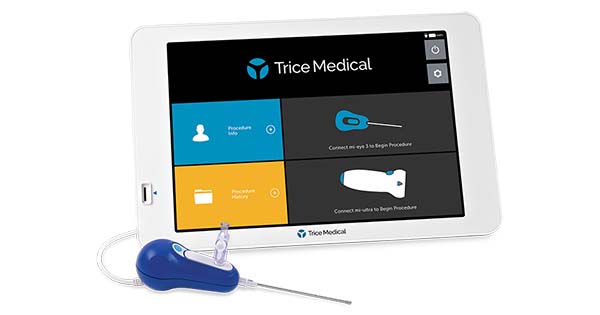
Trice Medical received FDA 510(k) Clearance for 25-degree mi-eye 3 needlescope™ with horizon leveling technology, offering unparalleled views and maneuverability in arthroscopic and endoscopic procedures from a single-use, single-hand device. Used with the proprietary high-resolution mi-tablet™ 3, the technology takes minimally invasive operative and diagnostic capabilities in small joints and patient outcomes to a new level.
The 25-degree mi-eye 3 needlescope and the high-resolution mi-tablet 3 platform integrates the latest technology with 8-in-1 capabilities: image horizon leveling, high-resolution image sensor, integrated camera with LED illumination, single-hand operation, image management, seamless workflow with hospital information management systems, the ability to manipulate the viewing angle with horizon preferences, and portability.
The new mi-eye 3 needlescope camera performs the same as a reusable but in a small disposable form. Its streamlined design eliminates undesirable aspects of existing reusable arthroscopes, including the two-handed light source and angle rotation requirements and multiple accident-prone cables. It also removes costly equipment, sterile processing, and other expenses.
Mark Foster, President and CEO of Trice Medical, said, “For decades, using an angled camera over a 0-degree camera has been the standard of care in arthroscopy. No physician would ever use a 0-degree reusable arthroscope in the operating room and now they don’t have to take a step back in quality just to use a disposable camera either. Our early data shows the potential to see over 16x more information with our angled camera vs. a 0-degree camera. This is another in a long list of worlds’ first innovations from Trice Medical.”
Source: Trice Medical
Trice Medical received FDA 510(k) Clearance for 25-degree mi-eye 3 needlescope™ with horizon leveling technology, offering unparalleled views and maneuverability in arthroscopic and endoscopic procedures from a single-use, single-hand device. Used with the proprietary high-resolution mi-tablet™ 3, the technology takes minimally invasive operative...
Trice Medical received FDA 510(k) Clearance for 25-degree mi-eye 3 needlescope™ with horizon leveling technology, offering unparalleled views and maneuverability in arthroscopic and endoscopic procedures from a single-use, single-hand device. Used with the proprietary high-resolution mi-tablet™ 3, the technology takes minimally invasive operative and diagnostic capabilities in small joints and patient outcomes to a new level.
The 25-degree mi-eye 3 needlescope and the high-resolution mi-tablet 3 platform integrates the latest technology with 8-in-1 capabilities: image horizon leveling, high-resolution image sensor, integrated camera with LED illumination, single-hand operation, image management, seamless workflow with hospital information management systems, the ability to manipulate the viewing angle with horizon preferences, and portability.
The new mi-eye 3 needlescope camera performs the same as a reusable but in a small disposable form. Its streamlined design eliminates undesirable aspects of existing reusable arthroscopes, including the two-handed light source and angle rotation requirements and multiple accident-prone cables. It also removes costly equipment, sterile processing, and other expenses.
Mark Foster, President and CEO of Trice Medical, said, “For decades, using an angled camera over a 0-degree camera has been the standard of care in arthroscopy. No physician would ever use a 0-degree reusable arthroscope in the operating room and now they don’t have to take a step back in quality just to use a disposable camera either. Our early data shows the potential to see over 16x more information with our angled camera vs. a 0-degree camera. This is another in a long list of worlds’ first innovations from Trice Medical.”
Source: Trice Medical

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







