
 Copy to clipboard
Copy to clipboard 
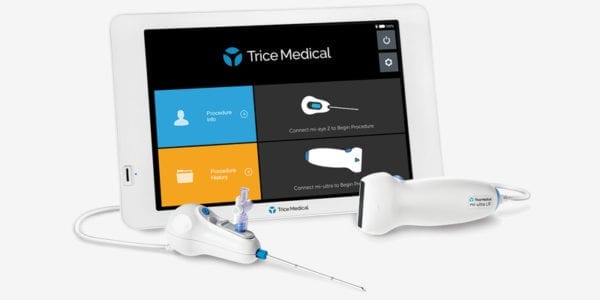
Trice Medical received CE Mark Approval and Health Canada approval for the mi-eye 2™ direct visualization tool, and announced completion of first cases in the regions.
mi-eye 2 received FDA 510(k) clearance in late 2016 and launched in the U.S. in 2017, where it’s now used in >200 settings. The technology is a handheld, single-use tool comprising a wide-angle camera lens embedded in a disposable needle. Physicians can complete a diagnosis on the spot in a clinic, under local anesthetic.
Sources: Trice Medical; ORTHOWORLD Inc.
Trice Medical received CE Mark Approval and Health Canada approval for the mi-eye 2™ direct visualization tool, and announced completion of first cases in the regions.
mi-eye 2 received FDA 510(k) clearance in late 2016 and launched in the U.S. in 2017, where it's now used in >200 settings. The technology is a handheld, single-use tool...
Trice Medical received CE Mark Approval and Health Canada approval for the mi-eye 2™ direct visualization tool, and announced completion of first cases in the regions.
mi-eye 2 received FDA 510(k) clearance in late 2016 and launched in the U.S. in 2017, where it’s now used in >200 settings. The technology is a handheld, single-use tool comprising a wide-angle camera lens embedded in a disposable needle. Physicians can complete a diagnosis on the spot in a clinic, under local anesthetic.
Sources: Trice Medical; ORTHOWORLD Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







