
 Copy to clipboard
Copy to clipboard 
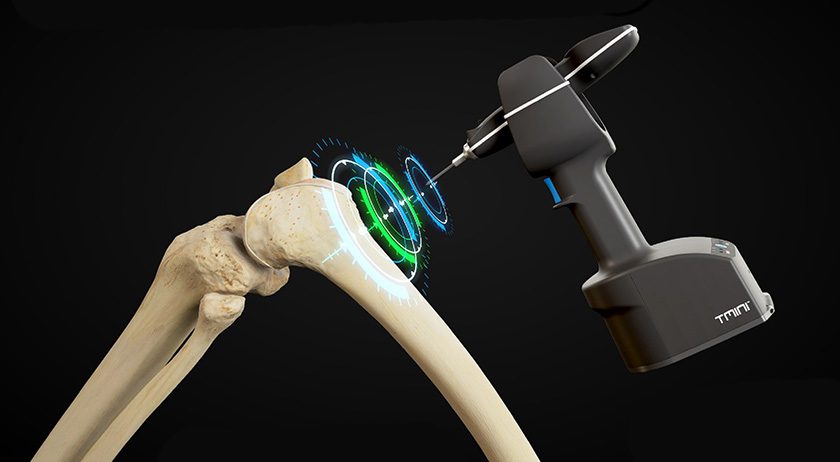
THINK Surgical announced that its TMINI Miniature Robotic System has been utilized in over 500 total knee arthroplasty (TKA) procedures in the United States.
The TMINI Miniature Robotic System initially received FDA 510(k) clearance in 2023 with a single implant partner. It has since received multiple clearances for the TMINI 1.1 Version Update that enables the TMINI PRO Workflow, enabling Positional Refinement and Optimization of the implant, and eight additional implant partners by November 2024.
With nine FDA 510(k)-cleared implant partners now available, THINK Surgical offers implant choice to surgeons and administrators. The system is commercially available throughout the United States.
“Achieving this 500-case milestone so quickly demonstrates the market’s eagerness to adopt our miniature, handheld, wireless robot,” said Stuart Simpson, President and Chief Executive Officer of THINK Surgical. “Surgeon and hospital staff using the TMINI System are reporting that they enjoy it because of its accuracy and efficiency in the OR. We are excited about our new customer pipeline and look forward to seeing many more successful TKA procedures completed with the TMINI Robot.”
Source: THINK Surgical, Inc.
THINK Surgical announced that its TMINI Miniature Robotic System has been utilized in over 500 total knee arthroplasty (TKA) procedures in the United States.
The TMINI Miniature Robotic System initially received FDA 510(k) clearance in 2023 with a single implant partner. It has since received multiple clearances for the TMINI 1.1 Version...
THINK Surgical announced that its TMINI Miniature Robotic System has been utilized in over 500 total knee arthroplasty (TKA) procedures in the United States.
The TMINI Miniature Robotic System initially received FDA 510(k) clearance in 2023 with a single implant partner. It has since received multiple clearances for the TMINI 1.1 Version Update that enables the TMINI PRO Workflow, enabling Positional Refinement and Optimization of the implant, and eight additional implant partners by November 2024.
With nine FDA 510(k)-cleared implant partners now available, THINK Surgical offers implant choice to surgeons and administrators. The system is commercially available throughout the United States.
“Achieving this 500-case milestone so quickly demonstrates the market’s eagerness to adopt our miniature, handheld, wireless robot,” said Stuart Simpson, President and Chief Executive Officer of THINK Surgical. “Surgeon and hospital staff using the TMINI System are reporting that they enjoy it because of its accuracy and efficiency in the OR. We are excited about our new customer pipeline and look forward to seeing many more successful TKA procedures completed with the TMINI Robot.”
Source: THINK Surgical, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







