
 Copy to clipboard
Copy to clipboard 
Update, 12/16/20: The system is slated for U.S. launch in early 2021.
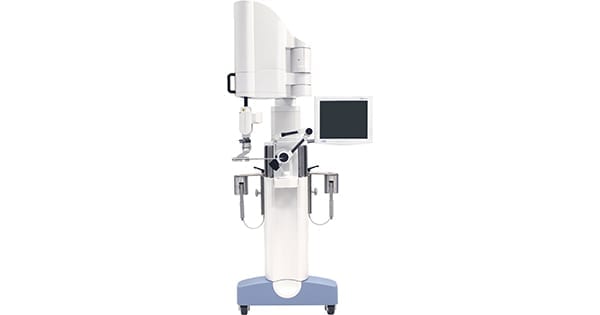
THINK Surgical was granted FDA 510(k) clearance for the second generation of the TSolution One Total Knee Application, an active robot for total knee replacement that provides fully automated bone preparation. The new system offers surgeons their choice of implant and features upgrades to the current system, including an enhanced pre-surgical planning user interface, quick-change tooling, improved surgeon accessories and advanced bone model generation.
The first-generation device received FDA 510(k) clearance in late 2019.
TSolution One Total Knee Application comprises the TPLAN® 3D pre-surgical planning workstation and TCAT®, the active robot that helps the surgeon execute the patient’s preoperative plan. Several changes to the hardware and software enhance system efficiency, flexibility and ease.
“The ongoing evolution of the TSolution One Total Knee Application is a testament to THINK Surgical’s dedication and investment in advancing the use of robot technology in the orthopedic setting,” said Jay Yang, acting CEO, THINK Surgical. “The versatile, open platform provides surgeons with the flexibility of using a variety of implants, while offering hospitals and ambulatory surgery centers a sustainable, high throughput system for their ever-increasing total knee replacement procedures.”
Update, 12/16/20: The system is slated for U.S. launch in early 2021.
THINK Surgical was granted FDA 510(k) clearance for the second generation of the TSolution One Total Knee Application, an active robot for total knee replacement that provides fully automated bone preparation. The new system offers surgeons their choice of implant...
Update, 12/16/20: The system is slated for U.S. launch in early 2021.
THINK Surgical was granted FDA 510(k) clearance for the second generation of the TSolution One Total Knee Application, an active robot for total knee replacement that provides fully automated bone preparation. The new system offers surgeons their choice of implant and features upgrades to the current system, including an enhanced pre-surgical planning user interface, quick-change tooling, improved surgeon accessories and advanced bone model generation.
The first-generation device received FDA 510(k) clearance in late 2019.
TSolution One Total Knee Application comprises the TPLAN® 3D pre-surgical planning workstation and TCAT®, the active robot that helps the surgeon execute the patient’s preoperative plan. Several changes to the hardware and software enhance system efficiency, flexibility and ease.
“The ongoing evolution of the TSolution One Total Knee Application is a testament to THINK Surgical’s dedication and investment in advancing the use of robot technology in the orthopedic setting,” said Jay Yang, acting CEO, THINK Surgical. “The versatile, open platform provides surgeons with the flexibility of using a variety of implants, while offering hospitals and ambulatory surgery centers a sustainable, high throughput system for their ever-increasing total knee replacement procedures.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







