
 Copy to clipboard
Copy to clipboard 
Theradaptive was awarded funding from the Maryland Stem Cell Research Fund (MSCRF) to support human clinical trials for its lead product, OsteoAdapt SP. OsteoAdapt SP is currently in Phase I/II clinical studies for transforaminal lumbar interbody spinal fusion (TLIF) to treat degenerative disc disease, spondylolisthesis and retrolisthesis.
Theradaptive was granted an Investigational Device Exemption in January 2024 by FDA to begin its human clinical trial. This $1 million award from the MSCRF Clinical Program will enable Theradaptive to expand its OASIS human clinical study to sites in Maryland. Theradaptive also holds three Breakthrough Medical Device designations for various spine indications, including TLIF, ALIF and PLF.
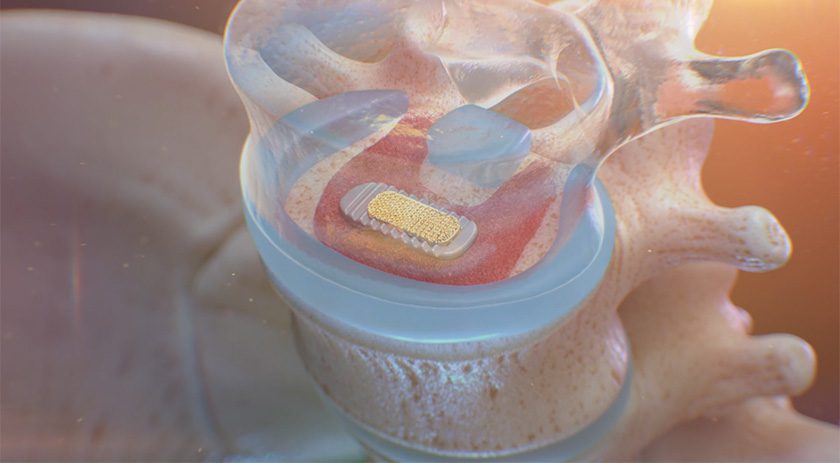
OsteoAdapt SP is a biologic-enhanced implant designed to stimulate anatomically precise local bone growth and promote rapid fusion following spinal surgery. It combines a proprietary protein called AMP2 that activates a patient’s own stem cells with a resorbable scaffold implant. This implant remodels into bone and completely resorbs, leaving no trace behind. This technology ushers in the next generation of regenerative therapeutics compared to the current standard of care by mitigating side effects and significantly improving safety and efficacy over traditional bone grafts and biologics.
Theradaptive was spun out of the Massachusetts Institute of Technology in 2017 to commercialize a platform that immobilizes therapeutic proteins on implantable biomaterials. The company’s near-term focus is on regenerative treatments for musculoskeletal conditions and spinal fusion surgery.
Clinical sites investigating OsteoAdapt SP in TLIF procedures are currently enrolling patients across the U.S., including Maryland, and in Australia.
Theradaptive plans to file for marketing authorization with the U.S. Food and Drug Administration following successful completion of pivotal clinical studies.
“This funding will benefit us greatly as we work toward making this revolutionary therapy available to patients in need, putting Maryland at the forefront of innovation in regenerative bone repair,” said Jonathan Elsner, PhD, Vice President of Clinical Operations. “We appreciate that MSCRF recognizes the importance of this program and look forward to dosing the first patient in the coming months.”
“We are so grateful to the Maryland Stem Cell Research Fund for this generous support as we take OsteoAdapt SP through clinical development,” said Luis Alvarez, PhD, CEO and Founder of Theradaptive. “This grant will expand our ability to provide patients with limited options a much better alternative by accelerating the development of this ground-breaking technology.”
Source: Theradaptive
Theradaptive was awarded funding from the Maryland Stem Cell Research Fund (MSCRF) to support human clinical trials for its lead product, OsteoAdapt SP. OsteoAdapt SP is currently in Phase I/II clinical studies for transforaminal lumbar interbody spinal fusion (TLIF) to treat degenerative disc disease, spondylolisthesis and retrolisthesis.
...
Theradaptive was awarded funding from the Maryland Stem Cell Research Fund (MSCRF) to support human clinical trials for its lead product, OsteoAdapt SP. OsteoAdapt SP is currently in Phase I/II clinical studies for transforaminal lumbar interbody spinal fusion (TLIF) to treat degenerative disc disease, spondylolisthesis and retrolisthesis.
Theradaptive was granted an Investigational Device Exemption in January 2024 by FDA to begin its human clinical trial. This $1 million award from the MSCRF Clinical Program will enable Theradaptive to expand its OASIS human clinical study to sites in Maryland. Theradaptive also holds three Breakthrough Medical Device designations for various spine indications, including TLIF, ALIF and PLF.
OsteoAdapt SP is a biologic-enhanced implant designed to stimulate anatomically precise local bone growth and promote rapid fusion following spinal surgery. It combines a proprietary protein called AMP2 that activates a patient’s own stem cells with a resorbable scaffold implant. This implant remodels into bone and completely resorbs, leaving no trace behind. This technology ushers in the next generation of regenerative therapeutics compared to the current standard of care by mitigating side effects and significantly improving safety and efficacy over traditional bone grafts and biologics.
Theradaptive was spun out of the Massachusetts Institute of Technology in 2017 to commercialize a platform that immobilizes therapeutic proteins on implantable biomaterials. The company’s near-term focus is on regenerative treatments for musculoskeletal conditions and spinal fusion surgery.
Clinical sites investigating OsteoAdapt SP in TLIF procedures are currently enrolling patients across the U.S., including Maryland, and in Australia.
Theradaptive plans to file for marketing authorization with the U.S. Food and Drug Administration following successful completion of pivotal clinical studies.
“This funding will benefit us greatly as we work toward making this revolutionary therapy available to patients in need, putting Maryland at the forefront of innovation in regenerative bone repair,” said Jonathan Elsner, PhD, Vice President of Clinical Operations. “We appreciate that MSCRF recognizes the importance of this program and look forward to dosing the first patient in the coming months.”
“We are so grateful to the Maryland Stem Cell Research Fund for this generous support as we take OsteoAdapt SP through clinical development,” said Luis Alvarez, PhD, CEO and Founder of Theradaptive. “This grant will expand our ability to provide patients with limited options a much better alternative by accelerating the development of this ground-breaking technology.”
Source: Theradaptive

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.