
 Copy to clipboard
Copy to clipboard 
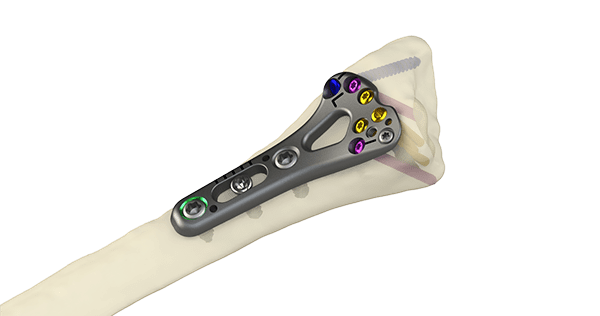
The Orthopaedic Implant Company announced FDA 510(k) clearance and U.S. launch of its DRPx wrist fracture plate. DRPx is the only distal radius plating system that features an enhanced ergonomic design to meet the technique preferences of surgeons while significantly increasing cost savings to help improve the financial viability of ambulatory surgery centers and hospitals.
Designed with Type II anodized titanium, the DRPx features:
- Pre-contoured anatomic plates
- Low-profile plate and screw design to minimize soft tissue impingement
- Variable angle and fixed angle locking screws, locking pegs, and non-locking screws provide optionality for any technique
- Distal window for graft and/or fracture visualization
- Indicator lines for improved guidance and adjustment
- A full spectrum of targeting instrumentation that facilitates precise and fast drilling trajectories
Source: The Orthopaedic Implant Company
The Orthopaedic Implant Company announced FDA 510(k) clearance and U.S. launch of its DRPx wrist fracture plate. DRPx is the only distal radius plating system that features an enhanced ergonomic design to meet the technique preferences of surgeons while significantly increasing cost savings to help improve the financial viability of ambulatory...
The Orthopaedic Implant Company announced FDA 510(k) clearance and U.S. launch of its DRPx wrist fracture plate. DRPx is the only distal radius plating system that features an enhanced ergonomic design to meet the technique preferences of surgeons while significantly increasing cost savings to help improve the financial viability of ambulatory surgery centers and hospitals.
Designed with Type II anodized titanium, the DRPx features:
- Pre-contoured anatomic plates
- Low-profile plate and screw design to minimize soft tissue impingement
- Variable angle and fixed angle locking screws, locking pegs, and non-locking screws provide optionality for any technique
- Distal window for graft and/or fracture visualization
- Indicator lines for improved guidance and adjustment
- A full spectrum of targeting instrumentation that facilitates precise and fast drilling trajectories
Source: The Orthopaedic Implant Company

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







