
 Copy to clipboard
Copy to clipboard 
Approximately 30 million people in the U.S. suffer from chronic tendon pain, and half of those have little or no relief from physical therapy or medication, according to studies cited by the Centers for Disease Control and Prevention. When medication or therapy fail to ease pain, the next step is often tenotomy procedure in an operating room.
To ease symptoms, often a manual approach is employed by applying a needle through the skin and into the tendon, triggering a healing response. The procedure, called percutaneous needle tenotomy, is a controlled re-injury using a standard syringe needle to restart the natural healing cycle. The procedure is minimally invasive. However, results can be inconsistent because its effectiveness is determined by tactile feedback, which requires a long learning process by the healthcare provider. Technology from the startup TendoNova aims to increase procedure effectiveness while reducing the cost and improving the patient’s and physician’s experiences.
How It Works
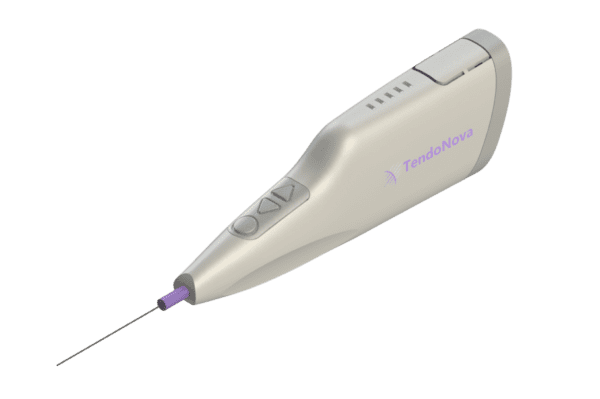
The TendoNova Ocelot system expands upon the percutaneous tenotomy technique with a compact, motor-driven tool to perform the same kind of treatment, while simultaneously providing real-time data feedback and data capture to show the procedure’s effectiveness. Ocelot is used during a minimally invasive procedure that can be performed with local anesthetic under ultrasound guidance in a clinician’s office.
“The current methods for treating chronic tendon pain are expensive, complex and have variable outcomes,” said Roy Wallen, CEO of TendoNova Corporation. “Moreover, these systems are usually deployed in a surgery center, which has higher operating costs. Our approach is to provide a simple, effective tool that can be easily adopted in a physician’s office.”
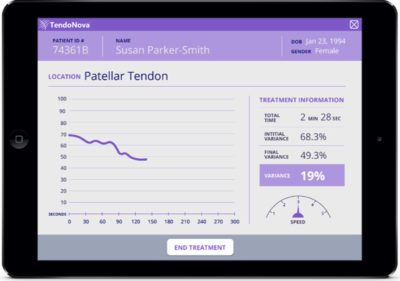
TendoNova System Display Unit
The Ocelot system consists of a single-use probe specialized for percutaneous tenotomy, with a reusable, chargeable driver. Integrating into the clinical workflow, it connects with a display unit to provide real-time measurement of the procedure’s effectiveness.
The key innovation with the TendoNova technology is the ability to present instant information to the clinician on the state of the procedure. The data are then stored for future reference and analysis, giving physicians and patients the ability to track long-term improvements.
Benefits to Patient and Physician
As the cost of healthcare continues to increase, TendoNova says that its competitive advantage is offering a low-cost solution that can be used in a low-cost setting.
The ability to perform the procedure in a physician’s office could provide improved clinical outcomes and patient experience, as there is no need for operating rooms and a lower risk for infection than an open procedure would have. And while biological therapeutics are also useful in treating tendinopathy, the outcomes have been variable and without reimbursement.
Ultrasound is increasingly affordable and sophisticated, allowing soft tissue pathologies to be identified and targeted in-office with local anesthesia through a quicker procedure.
Going to Market
The 30 million people living with chronic tendon pain in the U.S. include athletes and active young people. The NFL has even recognized TendoNova’s approach. The company was one of five chosen to present as a finalist for the 2019 NFL’s “1st and Future” competition, beating out more than 100 applicants from around the world.
“We estimate that in the U.S. alone there is a $1.2 billion market opportunity for our technology,” Wallen said. “Initial estimates show that the European market is approximately the same size as in the U.S.”
The go-to-market version of the Ocelot system has been completed, and the company is performing testing and validation needed for regulatory submission.
Primary, directly competing technologies have been developed by Tenex Health and HydroCision. Other indirectly competing technologies are offered by companies such as DePuy Mitek Sports Medicine, Arthrex, Terumo BCT and Juventix.
Approximately 30 million people in the U.S. suffer from chronic tendon pain, and half of those have little or no relief from physical therapy or medication, according to studies cited by the Centers for Disease Control and Prevention. When medication or therapy fail to ease pain, the next step is often tenotomy procedure in an operating room.
...
Approximately 30 million people in the U.S. suffer from chronic tendon pain, and half of those have little or no relief from physical therapy or medication, according to studies cited by the Centers for Disease Control and Prevention. When medication or therapy fail to ease pain, the next step is often tenotomy procedure in an operating room.
To ease symptoms, often a manual approach is employed by applying a needle through the skin and into the tendon, triggering a healing response. The procedure, called percutaneous needle tenotomy, is a controlled re-injury using a standard syringe needle to restart the natural healing cycle. The procedure is minimally invasive. However, results can be inconsistent because its effectiveness is determined by tactile feedback, which requires a long learning process by the healthcare provider. Technology from the startup TendoNova aims to increase procedure effectiveness while reducing the cost and improving the patient’s and physician’s experiences.
How It Works
The TendoNova Ocelot system expands upon the percutaneous tenotomy technique with a compact, motor-driven tool to perform the same kind of treatment, while simultaneously providing real-time data feedback and data capture to show the procedure’s effectiveness. Ocelot is used during a minimally invasive procedure that can be performed with local anesthetic under ultrasound guidance in a clinician’s office.
“The current methods for treating chronic tendon pain are expensive, complex and have variable outcomes,” said Roy Wallen, CEO of TendoNova Corporation. “Moreover, these systems are usually deployed in a surgery center, which has higher operating costs. Our approach is to provide a simple, effective tool that can be easily adopted in a physician’s office.”

TendoNova System Display Unit
The Ocelot system consists of a single-use probe specialized for percutaneous tenotomy, with a reusable, chargeable driver. Integrating into the clinical workflow, it connects with a display unit to provide real-time measurement of the procedure’s effectiveness.
The key innovation with the TendoNova technology is the ability to present instant information to the clinician on the state of the procedure. The data are then stored for future reference and analysis, giving physicians and patients the ability to track long-term improvements.
Benefits to Patient and Physician
As the cost of healthcare continues to increase, TendoNova says that its competitive advantage is offering a low-cost solution that can be used in a low-cost setting.
The ability to perform the procedure in a physician’s office could provide improved clinical outcomes and patient experience, as there is no need for operating rooms and a lower risk for infection than an open procedure would have. And while biological therapeutics are also useful in treating tendinopathy, the outcomes have been variable and without reimbursement.
Ultrasound is increasingly affordable and sophisticated, allowing soft tissue pathologies to be identified and targeted in-office with local anesthesia through a quicker procedure.
Going to Market
The 30 million people living with chronic tendon pain in the U.S. include athletes and active young people. The NFL has even recognized TendoNova’s approach. The company was one of five chosen to present as a finalist for the 2019 NFL’s “1st and Future” competition, beating out more than 100 applicants from around the world.
“We estimate that in the U.S. alone there is a $1.2 billion market opportunity for our technology,” Wallen said. “Initial estimates show that the European market is approximately the same size as in the U.S.”
The go-to-market version of the Ocelot system has been completed, and the company is performing testing and validation needed for regulatory submission.
Primary, directly competing technologies have been developed by Tenex Health and HydroCision. Other indirectly competing technologies are offered by companies such as DePuy Mitek Sports Medicine, Arthrex, Terumo BCT and Juventix.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.







