
 Copy to clipboard
Copy to clipboard 
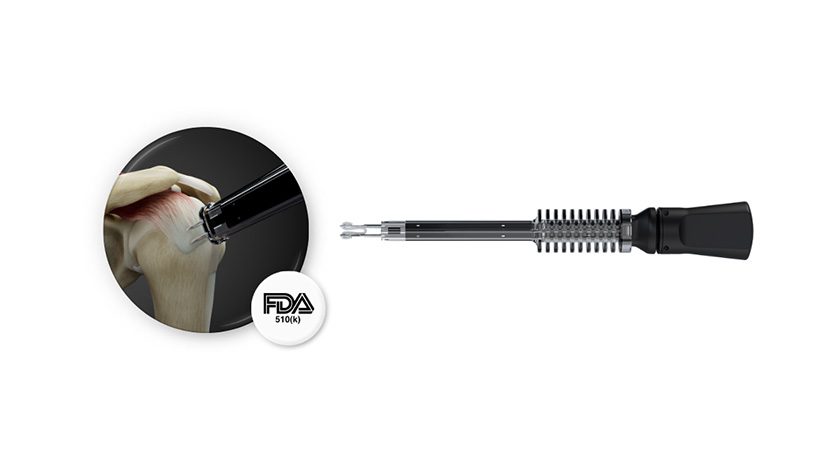
SutureTech was granted FDA 510(k) clearance to market RapidFix, an All-Suture Dual Anchor System. RapidFix is intended for fixation of soft tissue to bone in a variety of orthopedic procedures. Limited launch will occur within 4Q25.
RapidFix is a 100% suture-based internal fixation device engineered to create secure tendon-to-bone repairs while reducing procedural steps and surgical complexity. Developed through eight years of surgeon-led collaboration, the device has been validated through preclinical testing for safety and performance.
“Achieving FDA clearance for RapidFix is a defining moment for SutureTech,” said Dr. Oke Anakwenze, Founder & CEO of SutureTech and Chief of Shoulder Surgery at Duke University. “The device was designed to streamline surgical steps while maintaining strong, reliable fixation. We believe RapidFix offers value for surgeons seeking reproducibility—and for patients who benefit from more consistent repairs.”
Source: SutureTech
SutureTech was granted FDA 510(k) clearance to market RapidFix, an All-Suture Dual Anchor System. RapidFix is intended for fixation of soft tissue to bone in a variety of orthopedic procedures. Limited launch will occur within 4Q25.
RapidFix is a 100% suture-based internal fixation device engineered to create secure tendon-to-bone repairs...
SutureTech was granted FDA 510(k) clearance to market RapidFix, an All-Suture Dual Anchor System. RapidFix is intended for fixation of soft tissue to bone in a variety of orthopedic procedures. Limited launch will occur within 4Q25.
RapidFix is a 100% suture-based internal fixation device engineered to create secure tendon-to-bone repairs while reducing procedural steps and surgical complexity. Developed through eight years of surgeon-led collaboration, the device has been validated through preclinical testing for safety and performance.
“Achieving FDA clearance for RapidFix is a defining moment for SutureTech,” said Dr. Oke Anakwenze, Founder & CEO of SutureTech and Chief of Shoulder Surgery at Duke University. “The device was designed to streamline surgical steps while maintaining strong, reliable fixation. We believe RapidFix offers value for surgeons seeking reproducibility—and for patients who benefit from more consistent repairs.”
Source: SutureTech

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







