Copy to clipboard
Copy to clipboard 
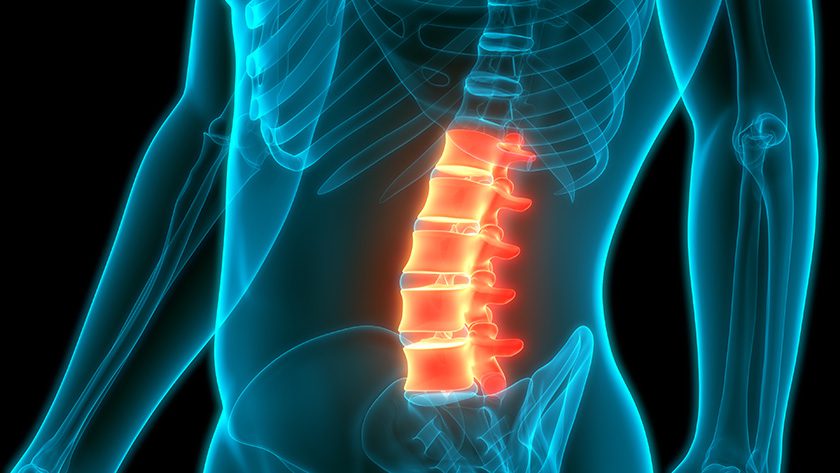
SurGenTec introduced the TiLINK-INSITE Sacroiliac Joint Fusion System. Now available in a sterile, surgery-ready kit, INSITE has already been successfully utilized in initial procedures performed across multiple U.S. sites.
The INSITE kit features a complete set of single-use, sterile instruments paired with a 3D-printed titanium implant. By eliminating the need for traditional sterilization, this all-in-one sterile solution streamlines surgical preparation and improves procedural efficiency.
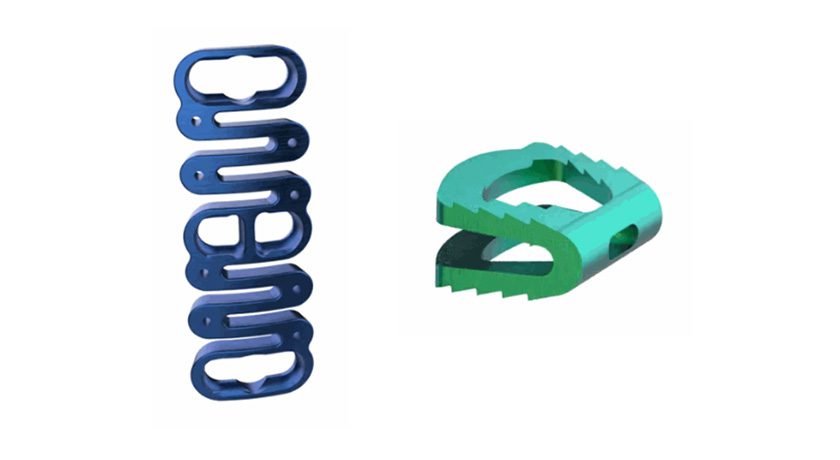
The implant incorporates proprietary nano-surface technology to encourage bone-on-implant growth and promote lasting biologic fixation. A large central graft window enables significant graft volume for robust osteointegration across the ilium and sacrum. The implant’s integrated trellis architecture delivers structural strength and open porosity to support bone in-growth throughout the device. Threaded insertion eliminates the need for malleting, minimizing surgical trauma and enabling a smooth, controlled placement through a single small incision—roughly the size of a quarter.
SurGenTec plans to make its entire SI joint fusion portfolio available in sterile kit formats by the end of 2025.
“INSITE has been engineered with both patients and physicians in mind,” said Travis Greenhalgh, CEO of SurGenTec. “We’re offering a system that not only simplifies the procedure with a complete, sterile, single-use kit but also provides the greatest opportunity for long-term fusion. The strong feedback we’ve received from early adopters validates our approach and furthers our mission to transform care in SI joint fusion.”
Source: SurGenTec
SurGenTec introduced the TiLINK-INSITE Sacroiliac Joint Fusion System. Now available in a sterile, surgery-ready kit, INSITE has already been successfully utilized in initial procedures performed across multiple U.S. sites.
The INSITE kit features a complete set of single-use, sterile instruments paired with a 3D-printed titanium implant. By...
SurGenTec introduced the TiLINK-INSITE Sacroiliac Joint Fusion System. Now available in a sterile, surgery-ready kit, INSITE has already been successfully utilized in initial procedures performed across multiple U.S. sites.
The INSITE kit features a complete set of single-use, sterile instruments paired with a 3D-printed titanium implant. By eliminating the need for traditional sterilization, this all-in-one sterile solution streamlines surgical preparation and improves procedural efficiency.
The implant incorporates proprietary nano-surface technology to encourage bone-on-implant growth and promote lasting biologic fixation. A large central graft window enables significant graft volume for robust osteointegration across the ilium and sacrum. The implant’s integrated trellis architecture delivers structural strength and open porosity to support bone in-growth throughout the device. Threaded insertion eliminates the need for malleting, minimizing surgical trauma and enabling a smooth, controlled placement through a single small incision—roughly the size of a quarter.
SurGenTec plans to make its entire SI joint fusion portfolio available in sterile kit formats by the end of 2025.
“INSITE has been engineered with both patients and physicians in mind,” said Travis Greenhalgh, CEO of SurGenTec. “We’re offering a system that not only simplifies the procedure with a complete, sterile, single-use kit but also provides the greatest opportunity for long-term fusion. The strong feedback we’ve received from early adopters validates our approach and furthers our mission to transform care in SI joint fusion.”
Source: SurGenTec

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.