
 Copy to clipboard
Copy to clipboard 
SurGenTec received FDA 510(k) clearance to market the 3D GraftRasp System™, including key spine indications. SurGenTec plans to release iterations for other general orthopedic applications such as large joints and the foot/ankle.
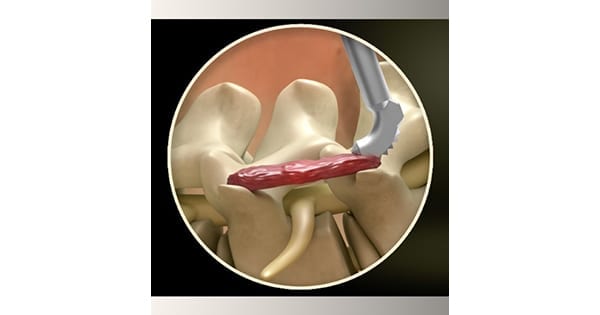
GraftRasp is the only device to receive FDA clearance that allows for both decortication of bone and the delivery of autograft, allograft or synthetic bone graft. It is approved for general orthopedic use and spine procedures. The 3D GraftRasp System is suitable for posterolateral or intertransverse lumbar fusions, and allows physicians to easily access the anatomy while making less invasive incisions, with the same standard of care as with an open lumbar fusion.
The system includes straight or curved rasps, specific to the surgical site anatomy. The rasps feature disposable foot plates with conical teeth for optimal decortication. 3D GraftRasp is intended for use with the GraftGun® delivery system for controlled bone graft delivery to the surgical site, and was designed to complement the GraftRasp instrument and GraftGun.
GraftGun tubes are pre-loaded with FDA-cleared synthetic OsteoFlo® NanoPutty®, ViMax® fiber cellular matrix or Ossify™ DBM putty and Ossify™ cortical fibers, and can also be used with the surgeon’s choice of bone graft.
“FDA clearance of the 3D GraftRasp System expands our treatment modalities in the spine market. This is an exciting time for our company and for physicians who need intuitive instruments to treat their patients,” said Andrew Shoup, SurGenTec’s COO.
SurGenTec received FDA 510(k) clearance to market the 3D GraftRasp System™, including key spine indications. SurGenTec plans to release iterations for other general orthopedic applications such as large joints and the foot/ankle.
GraftRasp is the only device to receive FDA clearance that allows for both decortication of bone and the...
SurGenTec received FDA 510(k) clearance to market the 3D GraftRasp System™, including key spine indications. SurGenTec plans to release iterations for other general orthopedic applications such as large joints and the foot/ankle.
GraftRasp is the only device to receive FDA clearance that allows for both decortication of bone and the delivery of autograft, allograft or synthetic bone graft. It is approved for general orthopedic use and spine procedures. The 3D GraftRasp System is suitable for posterolateral or intertransverse lumbar fusions, and allows physicians to easily access the anatomy while making less invasive incisions, with the same standard of care as with an open lumbar fusion.
The system includes straight or curved rasps, specific to the surgical site anatomy. The rasps feature disposable foot plates with conical teeth for optimal decortication. 3D GraftRasp is intended for use with the GraftGun® delivery system for controlled bone graft delivery to the surgical site, and was designed to complement the GraftRasp instrument and GraftGun.
GraftGun tubes are pre-loaded with FDA-cleared synthetic OsteoFlo® NanoPutty®, ViMax® fiber cellular matrix or Ossify™ DBM putty and Ossify™ cortical fibers, and can also be used with the surgeon’s choice of bone graft.
“FDA clearance of the 3D GraftRasp System expands our treatment modalities in the spine market. This is an exciting time for our company and for physicians who need intuitive instruments to treat their patients,” said Andrew Shoup, SurGenTec’s COO.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







