
 Copy to clipboard
Copy to clipboard 
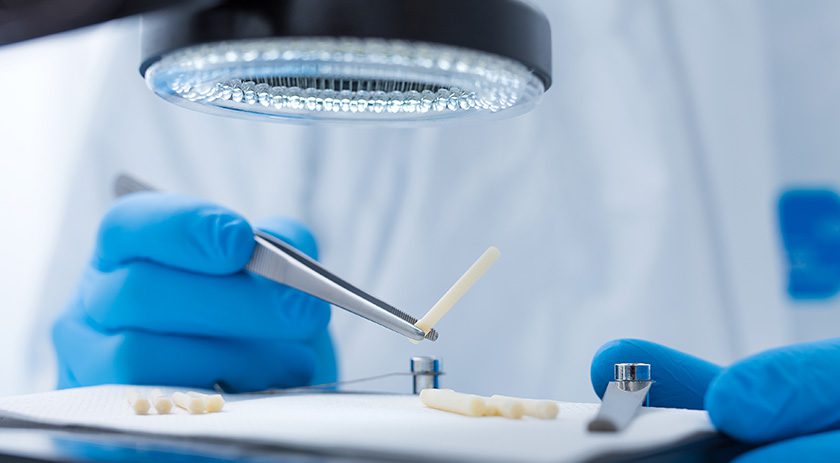
Surgebright commenced U.S. launch of Shark Screw, the first cortical allograft screw available on the market. The product remodels into the patient’s own bone (natural creeping substitution), thereby eliminating the need for hardware removal surgery and reducing the risk of complications.
Made from donor material, the screw undergoes a validated sterilization process that maintains the natural scaffold of the bone. It can be used to treat a wide range of conditions, with more than 70 indications for use in both lower and upper extremities, including osteosynthesis, fractures, non-unions and osteochondritis dissecans.
Shark Screw combines biological integration with mechanical stabilization and supports long-term healing by integrating into the patient’s bone structure. According to recent data published (Labmayr et al., 2024), the efficacy of the Shark Screw in promoting bone healing and reducing complications, when it comes to treat non-unions, has been well-documented.
“We are excited to bring Shark Screw to the U.S. market,” said Lukas Pastl, CEO of Surgebright Inc. “Since 2016, Shark Screw has been available in Europe, including Austria, Germany, Spain, and Switzerland, where thousands of patients have benefited from this innovative product. We are witnessing a significant shift towards a non-metal approach in the USA, which presents a tremendous opportunity for Shark Screw.”
Source: Surgebright Inc.
Surgebright commenced U.S. launch of Shark Screw, the first cortical allograft screw available on the market. The product remodels into the patient’s own bone (natural creeping substitution), thereby eliminating the need for hardware removal surgery and reducing the risk of complications.
Made from donor material, the screw undergoes a...
Surgebright commenced U.S. launch of Shark Screw, the first cortical allograft screw available on the market. The product remodels into the patient’s own bone (natural creeping substitution), thereby eliminating the need for hardware removal surgery and reducing the risk of complications.
Made from donor material, the screw undergoes a validated sterilization process that maintains the natural scaffold of the bone. It can be used to treat a wide range of conditions, with more than 70 indications for use in both lower and upper extremities, including osteosynthesis, fractures, non-unions and osteochondritis dissecans.
Shark Screw combines biological integration with mechanical stabilization and supports long-term healing by integrating into the patient’s bone structure. According to recent data published (Labmayr et al., 2024), the efficacy of the Shark Screw in promoting bone healing and reducing complications, when it comes to treat non-unions, has been well-documented.
“We are excited to bring Shark Screw to the U.S. market,” said Lukas Pastl, CEO of Surgebright Inc. “Since 2016, Shark Screw has been available in Europe, including Austria, Germany, Spain, and Switzerland, where thousands of patients have benefited from this innovative product. We are witnessing a significant shift towards a non-metal approach in the USA, which presents a tremendous opportunity for Shark Screw.”
Source: Surgebright Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







