
 Copy to clipboard
Copy to clipboard 
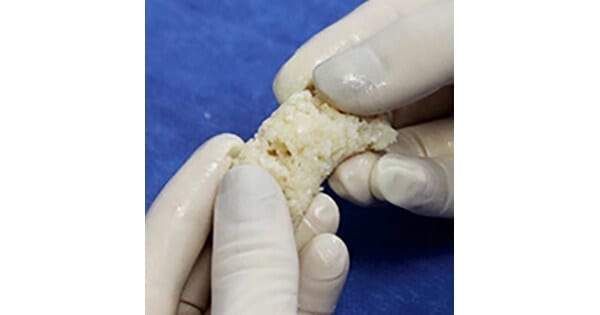
Surgalign commenced commercial U.S. launch and announced completion of the first surgery of ViBone Moldable bone graft, used to support spinal fusion.
ViBone Moldable, provided by Aziyo Biologics, is a next-generation viable cell bone matrix optimized to protect and preserve the health of native bone cells to potentially enhance new bone formation.
The product contains cancellous bone particles as well as demineralized cortical bone fibers and particles, delivering the necessary components for bone formation along with excellent handling and cohesive properties.
Terry Rich, President and Chief Executive Officer, said, “With this addition, we have strengthened Surgalign’s biologics portfolio to better serve our surgeon customers and patients. As we introduce new, advanced biologic products to our portfolio, we are well positioned to grow our business and provide better procedurally integrated products in each operating room.”
Surgalign commenced commercial U.S. launch and announced completion of the first surgery of ViBone Moldable bone graft, used to support spinal fusion.
ViBone Moldable, provided by Aziyo Biologics, is a next-generation viable cell bone matrix optimized to protect and preserve the health of native bone cells to potentially enhance new bone...
Surgalign commenced commercial U.S. launch and announced completion of the first surgery of ViBone Moldable bone graft, used to support spinal fusion.
ViBone Moldable, provided by Aziyo Biologics, is a next-generation viable cell bone matrix optimized to protect and preserve the health of native bone cells to potentially enhance new bone formation.
The product contains cancellous bone particles as well as demineralized cortical bone fibers and particles, delivering the necessary components for bone formation along with excellent handling and cohesive properties.
Terry Rich, President and Chief Executive Officer, said, “With this addition, we have strengthened Surgalign’s biologics portfolio to better serve our surgeon customers and patients. As we introduce new, advanced biologic products to our portfolio, we are well positioned to grow our business and provide better procedurally integrated products in each operating room.”

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







