
 Copy to clipboard
Copy to clipboard 
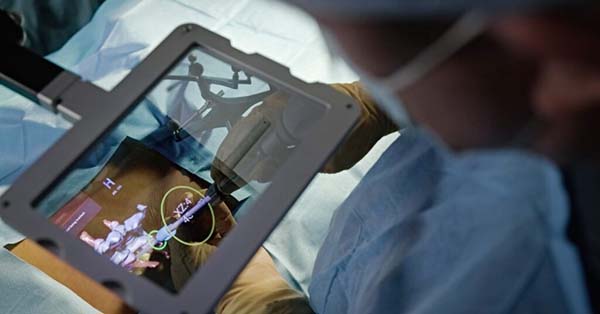
Surgalign received FDA 510(k) clearance to market its HOLO Portal surgical guidance system for use in lumbar spine procedures. The HOLO Portal system is the world’s first artificial intelligence (AI)-driven augmented reality (AR) guidance system for spine, and the first clinical application of Surgalign’s HOLO AI digital health platform.
HOLO Portal combines machine learning-based image guidance technology with AR, automated spine segmentation (i.e., anatomy recognition) and automated surgical planning utilizing proprietary AI software. Intraoperative images are autonomously processed by the AI system to create a patient-specific plan that is presented to the surgeon using the AR display.
Surgalign acquired Holo Surgical, developer of HOLO technology, in 2020.
“Receiving the initial clearance for the HOLO Portal system is a significant milestone and represents a critical step toward building the foundation of the digital surgery of the future. This system is designed to improve patient outcomes by delivering intelligent solutions to our customers, and we believe it is truly revolutionary,” said Terry Rich, Surgalign’s President and Chief Executive Officer. “With clearance in hand for our guidance application, our near-term focus is getting the platform into the hands of surgeons as we work towards a market release. While the current capabilities of the HOLO Portal system have the potential to offer a quantum leap in the way surgical procedures are performed, we have a much larger vision for our HOLO AI digital health platform across a variety of healthcare specialties and throughout the care continuum.”
Further, the company reported preliminary 4Q21 and full-year 2021 results:
- Preliminary global revenue for the quarter ended December 31, 2021, is expected to be within a range of $21.5 million to $21.9 million.
- Preliminary global revenue for the full year ended December 31, 2021, is expected to be within a range of $90.2 million to $90.6 million.
Source: Surgalign Holdings, Inc.
Surgalign received FDA 510(k) clearance to market its HOLO Portal surgical guidance system for use in lumbar spine procedures. The HOLO Portal system is the world’s first artificial intelligence (AI)-driven augmented reality (AR) guidance system for spine, and the first clinical application of Surgalign’s HOLO AI digital health platform.
HOLO...
Surgalign received FDA 510(k) clearance to market its HOLO Portal surgical guidance system for use in lumbar spine procedures. The HOLO Portal system is the world’s first artificial intelligence (AI)-driven augmented reality (AR) guidance system for spine, and the first clinical application of Surgalign’s HOLO AI digital health platform.
HOLO Portal combines machine learning-based image guidance technology with AR, automated spine segmentation (i.e., anatomy recognition) and automated surgical planning utilizing proprietary AI software. Intraoperative images are autonomously processed by the AI system to create a patient-specific plan that is presented to the surgeon using the AR display.
Surgalign acquired Holo Surgical, developer of HOLO technology, in 2020.
“Receiving the initial clearance for the HOLO Portal system is a significant milestone and represents a critical step toward building the foundation of the digital surgery of the future. This system is designed to improve patient outcomes by delivering intelligent solutions to our customers, and we believe it is truly revolutionary,” said Terry Rich, Surgalign’s President and Chief Executive Officer. “With clearance in hand for our guidance application, our near-term focus is getting the platform into the hands of surgeons as we work towards a market release. While the current capabilities of the HOLO Portal system have the potential to offer a quantum leap in the way surgical procedures are performed, we have a much larger vision for our HOLO AI digital health platform across a variety of healthcare specialties and throughout the care continuum.”
Further, the company reported preliminary 4Q21 and full-year 2021 results:
- Preliminary global revenue for the quarter ended December 31, 2021, is expected to be within a range of $21.5 million to $21.9 million.
- Preliminary global revenue for the full year ended December 31, 2021, is expected to be within a range of $90.2 million to $90.6 million.
Source: Surgalign Holdings, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







